Molarity of h2so4
Hey there! We receieved your request. Please choose valid name. Please Enter valid email.
Its density is 1. Hence, molality is:. What will be concentration of the solution in terms of molality and mole fraction? What will be the concentration in terms of molality and molar fraction? What will be concentration of the solution in terms of molarity and mole fraction?
Molarity of h2so4
.
We receieved your request. If density of solution is 1.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Mixtures and solutions. Definitions of solution, solute, and solvent. How molarity is used to quantify the concentration of solute, and how to calculate molarity. Key points. Mixtures with uniform composition are called homogeneous mixtures or solutions. Mixtures with non-uniform composition are heterogeneous mixtures.
Molarity of h2so4
Explain what changes and what stays the same when 1. What does it mean when we say that a mL sample and a mL sample of a solution of salt have the same molarity? In what ways are the two samples identical? In what ways are these two samples different? The two samples contain the same proportion of moles of salt to liters of solution, but have different numbers of actual moles. Calculate the number of moles and the mass of the solute in each of the following solutions:. There is about 1. What volume of a 1.
Goblin ep5 eng sub
The molality of a sulphuric acid solution is 0. Text Solution. View Solution. What is the molarity of H 2 SO 4 solution that has a density of 1. Register Now. If the density i In what volume ratio can 0. The molarity of the solution containing 2. The molality of A in H 2 O is: Equal moles of H 2 O and a solute of negligible molar mass are prese
This calculator can solve problems on the molarity or molar concentration of a solute in a solution. First, it can calculate the molar concentration of a solute given a solute chemical formula, the mass of the solute and the volume of the solution. Second, it can calculate the mass of a solute given a solute chemical formula, the volume of the solution and the desired molar concentration of a solute.
Test Series. In what volume ratio can 0. View all Questions ». The molarity of a solution containing 5. Forum Physical Chemistry What is the molarity of h2so4 solution that You will get reply from our expert in sometime. Studying in Grade 6th to 12th? To View your Question Click Here. Hey there! What will be the concentration in terms of molality and molar fraction? The density of HCl equal to 1. In what volume ratio can 0.

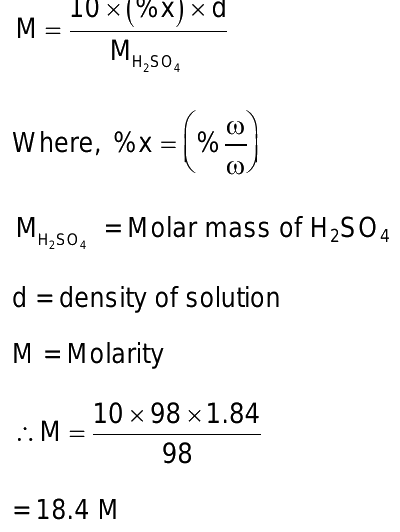
I consider, that you are mistaken.
This situation is familiar to me. Is ready to help.
Very useful piece