Magnesium core electrons
Be able to state how certain properties of atoms vary based on their relative position on the periodic table. One of the reasons the periodic table is so useful is because its structure allows us to qualitatively determine how some properties of the elements vary versus their position on the periodic table. The variation of properties versus position on the periodic table is called periodic trends. There is no other tool magnesium core electrons science that allows us to judge relative properties of a class of objects like this, magnesium core electrons, which makes the periodic table a very useful tool.
To begin our discussion of the trend in atomic radii lets consider the electron configuration for the elements in the third period, sodium through argon. To develop the next portion of the table we need to discuss two new terms; valence electrons and inner core electrons. Click in the picture on the right to start the clip of the lecture. So lets determine the number of valence electrons and inner core electrons for each of the elements in our table. Inner core electrons shield valence electrons from the nucleus. If we are on a valence electron looking back at the nucleus, the inner core electrons shield a portion of the nuclear charge from us. So the valence electron does not feel the attraction of all of the protons in the nucleus, but the attraction of an effective nuclear charge which is less than the total charge on the nucleus.
Magnesium core electrons
Periodic table as a reference is provided at the end of the article. Refer to the tutorial- What are valence and core electrons? How to determine a valence electron? The group number and atomic number is used to determine the valence electrons of an element. Carbon and Silicon belong to the same group 14 in the periodic table. All elements belonging to the same group in the periodic table will have the same number of valence electrons table A. The only difference will be their shell number due to the increase in atomic size. So, both Carbon and Silicon will have four valence electrons. The atomic number of Carbon is 6. Its electronic configuration after dividing the electrons into shells and orbitals is - 1s 2 2s 2 2p 2. The outermost shell is 2, containing four valence electrons 2s 2 2p 2 , and the two core electrons are in shell 1 1s 2. The atomic number of Silicon is Its electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 2. The outermost shell 3 contains four valence electrons 3s 2 3p 2 , and the remaining are Silicon's core electrons in shells 1 and 2 1s 2 2s 2 2p 6.
So, both Carbon and Silicon magnesium core electrons have four valence electrons. This results in a trend that in general the effective nuclear charge increases from left to right across any period of the periodic table. Functional Groups in Organic Chemistry What are functional groups?
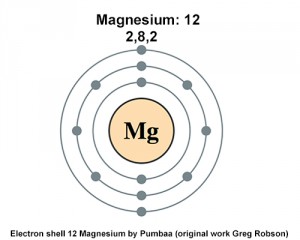
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Magnesium Mg In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom there are 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
The periodic table helps us to determine how some elements behave versus their position on the periodic chart. Property values are based on periodic trends, rather than position. Many of the regular trends are general. While there are instances where an opposite trend is apparent, a general trend emerges when considering if viewed across dozens or down whichever column of the table. Many of the periodic properties of atoms are dependent on electron configuration; in particular, the valence electrons and their adherence to the nucleus are at play with the numeclei. Atoms are classified differently based on their geographical location. To determine the number of core electrons, we must first determine the electron configuration of magnesium. Magnesium is a form of magnesium, so it has 12 protons and a Magnesium has two valence electrons. Magnesium is a form 12 and is classified in Group 2 of the Periodic Table.
Magnesium core electrons
Magnesium is essential for many biological processes, such as energy production, muscle contraction, nerve transmission, and bone formation. But how many valence electrons does magnesium have, and why does it matter? Valence electrons are the electrons in the outermost shell of an atom, which can participate in the formation of chemical bonds with other atoms. The number of valence electrons determines the chemical properties and reactivity of an element, as well as its ability to form compounds with different elements. Magnesium has 12 electrons, 12 protons and 12 neutrons that can vary. The first two shells are completely filled with eight electrons each, while the third shell has six electrons and the fourth shell has two electrons. The crucial part is that it has 2 valence electrons , both in the 3s orbital. This means that magnesium can lose these two electrons to achieve a stable configuration of eight valence electrons, which is the same as the noble gas neon.
Background diamond
Periodic Trends Learning Objectives 1. However, as you go across the periodic table and the electrons get drawn closer in, it takes more energy to remove an electron; as a result, IE increases:. Get Electronic Displacements in a Covalent Bond. Key Takeaways Certain properties—notably effective nuclear charge, atomic radius, IE, and EA—can be qualitatively understood by the positions of the elements on the periodic table. The outermost shell is 3 with two valence electrons 3s 2. So, the outermost shell is 2, containing five valence electrons. The atomic number of Silicon is Once you build the foundational understanding of Atoms, we will show how to calculate the mass number, average atomic mass, and molecular mass through step-by-step numerical examples. Magnesium and Calcium belong to Group number 2. Electronic Structure. Carbon and Silicon belong to the same group 14 in the periodic table. State the trends in IE as you go across and down the periodic table. Referring only to a periodic table and not to Figure 8. Be able to state how certain properties of atoms vary based on their relative position on the periodic table. Skip to main content.
In this article, I have discussed in detail how to easily write the complete electron configuration of magnesium. The total number of electrons in magnesium is twelve.
Magnesium and Calcium belong to Group number 2. Get Fundamentals of Organic Reactions. The effective nuclear charge is always less than the actual nuclear charge, and can be roughly estimated using the following equation:. So lets determine the number of valence electrons and inner core electrons for each of the elements in our table. Refer to the tutorial- What are valence and core electrons? As the principal quantum number n increases, the orbital size increases making the core electron clouds more spread out. Inner core electrons shield valence electrons from the nucleus. Get Physical Properties. Let's review what we've discussed and extend the idea of shielding and effective nuclear charge. State the trends in atomic radii as you go across and down the periodic table. For example, the following are the first three IEs for Mg, whose electron configuration is 1 s 2 2 s 2 2 p 6 3 s 2 :. The second IE is twice the first, which is not a surprise: the first IE involves removing an electron from a neutral atom, while the second one involves removing an electron from a positive ion. How to determine a valence electron?

I apologise, but, in my opinion, you are mistaken. I can prove it. Write to me in PM, we will talk.