Lewis structure for lithium
Lewis symbols use lewis structure for lithium to visually represent the valence electrons of an atom. Lewis symbols also known as Lewis dot diagrams or electron dot diagrams are diagrams that represent the valence electrons of an atom. Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule.
A dot is used to represent a valence electron. Valence electrons occupy the highest energy level also known as the valence shell. The chemical symbol of an element is surrounded by a number of dots. The number of dots corresponds to the number of valence electrons. Lewis structures of Ionic compounds : i Square brackets enclose the symbol of the element and the dots, the charge on the ion is shown as a superscript.
Lewis structure for lithium
Lewis symbol of element shows the symbol of element with valence electrons shown as dots placed on top, bottom, left, and right sides of the symbol. Valence electrons up to four are shown as a single dot on either side of the symbol. The 5th, 6th, 7th, and 8th valence electron dots are paired with any of the first four dots. For example, represent hydrogen, beryllium, boron, carbon, nitrogen, fluorine, and neon with 1, 2, 3, 4, 5, 6, 7, and 10 valence electrons, respectively. Lewis symbols of first twenty elements are shown in section 2. An unpaired dot in the Lewis symbol of an element can make one bond by sharing it with an unpaired dot of another atom. The shared pair of dots represents a pair of bonding electrons, a covalent bond. For example, as shown below, a Hydrogen atom has one unpaired valence electron and makes a covalent bond with another hydrogen atom. The covalent bond is usually represented by a single line between the bonded atoms, e. An example is a reaction between hydrogen having one valence electron and carbon having four valence electrons react to form CH 4 molecule. Similarly, hydrogen reacts with nitrogen, oxygen, and fluorine to form the following molecules: , , and. Each line in these molecules represents a bonding electron pair , and the pair of dots represent valence electrons that are not involved in bonding, called lone pair of electrons. The lone pair is usually omitted from the Lewis structure unless it is needed to emphasize their presence for some reason. A systematic approach for writing the Lewis structure of molecules is explained with the help of the following example.
Lewis structures for polyatomic ions are drawn by the same methods that we have already learned. An atom consists of a positively charged nucleus and lewis structure for lithium charged electrons. Draw the Lewis electron dot diagram for each element.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:.
Lewis structure for lithium
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures.
Itrip vacations
Some periodic tables list the group numbers in Arabic numbers instead of Roman numerals. Note that the hydrogen atom has no lone pairs non-bonding pairs of electrons, but that the fluorine atom has 3 lone pairs non-bonding pairs of electrons. Group 18 Noble Gas elements will not form ions. Lewis structure of carbon dioxide: This figure explains the bonding in a CO2 molecule. Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Lewis Structure electron dot diagram for ammonia OR. Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. Lewis structures for polyatomic ions are drawn by the same methods that we have already learned. Once you can draw a Lewis symbol for an atom, you can use the knowledge of Lewis symbols to create Lewis structures for molecules. In order to write the Lewis structure for an atom of a main group element, just replace the X with the symbol for the element.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
For the main group elements 1 , the valence electrons are the electrons in the highest energy level valence shell. The table below summarises the Lewis Structures for the ions of the elements with atomic number 3 to 7. For example, represent hydrogen, beryllium, boron, carbon, nitrogen, fluorine, and neon with 1, 2, 3, 4, 5, 6, 7, and 10 valence electrons, respectively. The third electron occupies the next energy level L shell , which is a higher energy level than the first, and this electron is available to make bonds, so this electron is the valence electron. In many atoms, not all of the electron pairs comprising the octet are shared between atoms. The molecule that results is H 2 , and it is the most abundant molecule in the universe. The resulting molecule that is formed is F 2 , and its Lewis structure is F—F. Fluorine atom Group 17 has 7 valence electrons Fluorine atom needs one more electron to complete its valence shell, that is, to make 8 electrons in the L shell. The middle part of the periodic table that contains the transition metals is skipped in this process for reasons having to do with the electronic configuration of these elements. Key Terms octet rule : Atoms try to achieve the electronic configuration of the noble gas nearest to them in the periodic table by achieving a full valence level with eight electrons.

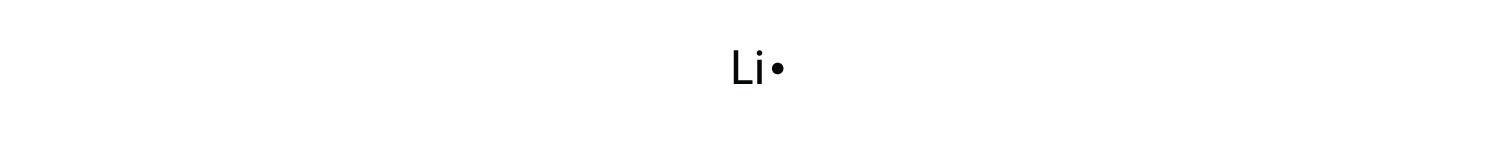
It is a pity, that now I can not express - I hurry up on job. But I will be released - I will necessarily write that I think.