Lewis dot structure for chcl3
Lewis structures are used to describe and visualize molecules. Lewis structures are used to show the bonds between atoms as well as the electrons surrounding certain atoms, lewis dot structure for chcl3. Note: The periodic table shows you how many valence electron each element has. Sometimes, we need to visualize a molecule.
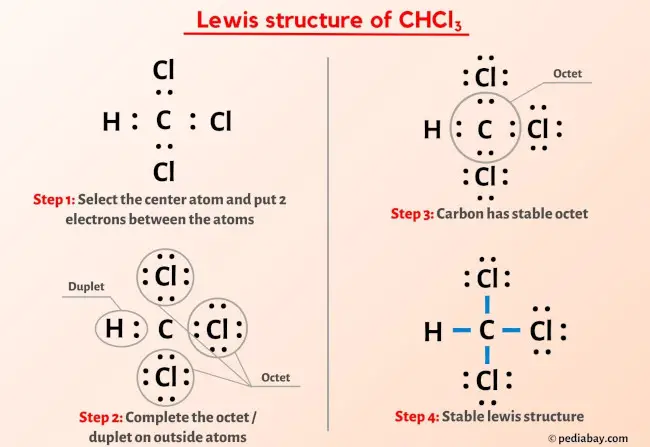
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom. Hydrogen atom has made a single bond with carbon atom and each chlorine atom has three lone pairs on their valence shell. As well, there are no charges on atoms in CHCl 3 lewis structure. When we draw a lewis structure, there are several guidelines to follow.
Lewis dot structure for chcl3
Get a free answer to a quick problem. Most questions answered within 4 hours. Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Draw the electron dot formula for The molecule of CHCl3. How many nonbonding electron pairs and bonding electron pairs are in the molecule of CHCl3? A 9 nonbonding electron pairs and 4 bonding electron pairs. B 6 nonbonding electron pairs and 4 bonding electron pairs. C 3 nonbonding electron pairs and 4 bonding electron pairs. D 3 nonbonding electron pairs and 3 bonding electron pairs. Add comment. Chlorines have 3 non-bonding pairs each.
The sulfur has a positive formal charge. VE is the total valence electrons from step two.
.
The chemical formula CHCl3 represents Chloroform. It is also known as Trichloromethane. Chloroform is a clear, colorless liquid that possesses a pleasant odor. It is nonflammable and is denser than water. It is generally prepared by the chlorination of methane. Chloroform first found use as an inhalation anesthetic in the 19th century. These days, it is produced industrially as a precursor to making Teflon. Chloroform also finds use as a refrigerant in many parts of the world. This refrigerant- HCFC22, contributes to the depletion of the ozone layer.
Lewis dot structure for chcl3
All the Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CHCl3. Here, the given molecule is CHCl3. In order to draw the lewis structure of CHCl3, first of all you have to find the total number of valence electrons present in the CHCl3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Virgin media wifi login
Moving forward, the Lewis structure will help us determine the VSEPR geometry , ideal bond angles and angle deviations. Carbon's four single bonds give it eight electrons. Chemistry Chemical Bonding. Add comment. The lewis structure of sulfur dioxide has sulfur double bonded to both oxygens. We have eighteen electrons remaining. Each oxygen has a complete octet. Eighteen from the three oxygens. In chloroform's molecular formula, the carbon is first, and there is only one atom of carbon. You've reached the end. You can turn lone pairs into another bond to reduce the number of formal charges. It is always the first atom listed. The carbon will have zero lone pairs. Try drawing the lewis structure for sulfur dioxide , SO2.
Trichloromethane, commonly known as chloroform, is a volatile organic compound in which one C-atom is covalently bonded to 3 Cl-atoms and 1 H-atom. At laboratory scale, it is prepared by chlorination of ethanol. Bleaching powder is often used as a chlorinating agent.
However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Hydrogen is on the remaining side. Boron only has three valence electrons. An example is borane BH3. There are eighteen valence electrons. The lewis structure of sulfite is sulfur with one double bond, two single bonds, and one lone pair. Add comment. Hydrogen has none. It is always the first atom listed. Lewis structures are used to show the bonds between atoms as well as the electrons surrounding certain atoms. One oxygen should have a single bond to sulfur and three lone pairs. Remember that, there are total of thirteen electron pairs. Wait, do I obey the octet rule or minimize the formal charges? Sulfur is the central atom.

I am sorry, that has interfered... I here recently. But this theme is very close to me. Is ready to help.
All above told the truth. We can communicate on this theme.