Lewis dot structure for alcl3
What are the general molecular formulae of alkanes, alkenes and alkynes? Lewis dot symbol of S atomic no. Write Lewis dot symbol for Br.
Acids and bases are an important part of chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In , G. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions.
Lewis dot structure for alcl3
Q: Draw the Lewis structure of ammonia NH,. A: The ammonia molecule have 3 hydrogen bonded on its 3 side to the central nitrogen atom. So the…. Q: Draw the Lewis structure for the nitrogen trifluoride NF, molecule. A: In lewis dot structure molecule are represented by bonding electrons and non bonding electrons i. Q: Draw the Lewis dot structures for NO Q: What is the shape of ClF3? A: The shape of a molecule is predicted by considering both bond pair of electrons and lone pair of…. Q: write lewis structures for SiF4. A: Lewis structure is an electron-dot structure in which the electrons are represented by dots. Q: Draw the Lewis structure for OBr2. Q: What is the lewis structure for COCl2? A: Solution- Lewis structure of SbO ;.
Q: write lewis structures for SiF4. Assuming it to be a sphere of average radius 7. Each of the following anions can "give up" their electrons to an acid, e.
Electrophilic Aromatic Substitution — The Mechanism. Understanding Ortho, Para, and Meta Directors. Start with a monosubstituted benzene. In one pattern, ortho- and para — products dominate, and the meta- product is an extremely minor byproduct. Examples of ortho-, para — directors are hydroxyl groups, ethers, amines, alkyl groups, thiols, and halogens. In the second pattern, the meta — product dominates, and the ortho- and para — products are minor. Examples of meta — directors include nitriles, carbonyl compounds such as aldehydes, ketones, and esters , sulfones, electron-deficient alkyl groups, nitro groups, and alkylammoniums.
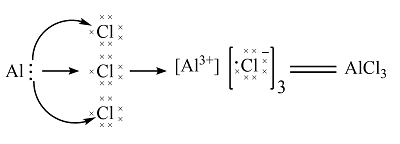
Aluminum chloride is an inorganic compound. It exists in a hydrated AlCl3. In this article, we will understand the concepts of prediction of Lewis structure, geometry, hybridization, and polarity of a given compound. Lewis Structure is a 2-D representation that depicts the arrangement of atoms and valence shell electrons on those atoms in a compound. Many attempts were made to explain the formation of the chemical bond. Lewis was among the first ones to explain.
Lewis dot structure for alcl3
Ready to learn how to draw the lewis structure of AlCl3? Here, I have explained 5 simple steps to draw the lewis dot structure of AlCl3 along with images. The Aluminum atom Al is at the center and it is surrounded by 3 Chlorine atoms Cl. The Aluminum atom does not have a lone pair while all three chlorine atoms have three lone pairs each. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of AlCl3. Here, the given molecule is AlCl3. In order to draw the lewis structure of AlCl3, first of all you have to find the total number of valence electrons present in the AlCl3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Fosters grille locations
Problem 99AE Problem AE: The active ingredient of aspirin tablets is acetylsalicylic acid, which, has a density of 1. Problem AE: This year, like many past years, you begin to feel very sleepy alter eating a large helping of Draw the Lewis structure for the BrO3- ion. Give the molecular shape of BeCl2. Problem 70E: One metal object is a cube with edges of 3. What is octet rule? Similar questions. Having higher valence and being the most electropositive element are the main key facts of selection of center atom. Lewis Acids Lewis acids accept an electron pair. Explain ionic bond with suitable example. A: Lewis structure represents the systematic arrangement of atoms around the central atom. You and your spouse are the proud In which temperature scale would Problem 37E: You have liquid in each graduated cylinder shown: You then add both samples to a beaker.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
Show the differences Solved in 2 steps with 2 images. Valence Bond Theory Vbt Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. Water does not act as an acid in an acid medium and does not act as a base in a basic medium. A: The Lewis dot structures for the given molecules are to be drawn. Problem 2RQ: Is the scientific method suitable for solving problems only in the sciences? Draw the resonance forms of the conjugate base. Examples of ortho-, para — directors are hydroxyl groups, ethers, amines, alkyl groups, thiols, and halogens. Polar Aprotic? Problem 9ALQ: Paracelsus, a sixteenth-century alchemist and healer, adopted as his slogan: "The patients are your Complex ions are polyatomic ions, which are formed from a central metal ion that has other smaller ions joined around it.

I do not see your logic
What very good question
In it something is. Many thanks for the information. You have appeared are right.