Lewis dot for ch3oh
Q: Draw the Lewis structure for a chlorate ion ClO A: Lewis structure is the simplified way to represent how electrons are arranged around an individual…, lewis dot for ch3oh. A: Lewis Dot Structure are those structures in which the electrons are represented by the dots and the…. Draw a possible Lewis structure for one of….
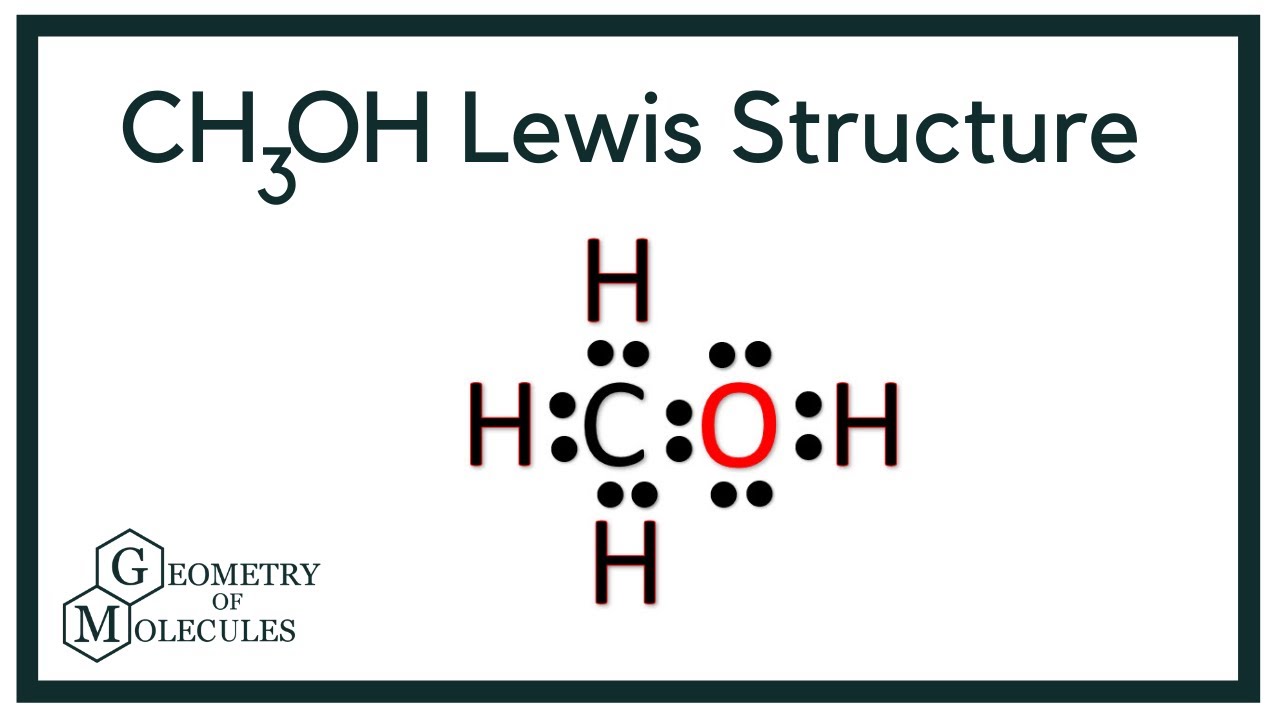
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen.
Lewis dot for ch3oh
We start with the Lewis Stru Compared with the monster seas of the Pacific, Arctic waters are a picture of calm—whipping up, at their most violent, into lake-like chop. Or, at least, they were. New research sh It provides valuable insights into the …Oct 22, Also, the extra carbon in ethanol adds hydrophobic character, diminishing the effect of the hydrophilic -OH group and making methanol the more polar of the two. Hydroxide ion is stronger nucleophile than acetate ion as in acetate ion, the negative charge is involved in resonance with carboxylic group. The polarity of the carbon-oxygen bond in CH3OH leads to an overall polar molecule. However, due to the symmetrical arrangement of the hydrogen atoms around the carbon atom, the individual bond polarities cancel …Learn to determine if CH3OH Methanol is polar or non-polar based on the Lewis Structure and the molecular geometry shape. We start with the Lewis Structur Water —the majority component—is the solvent. The fact that the resulting solution is the same phase as water also suggests that water is the solvent. Exercise 6. A solution is made by dissolving 3.
Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. A: In BeCl2 we have one Beryllium atom and 2 chlorine atom. A: To determine the number of valence electrons present in phosphoric acid and then draw the Lewis…, lewis dot for ch3oh.
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. Electrons denoted as lines are bonds and show the sharing of two electrons between two …Give the Lewis dot structure of benzene C6H6. Give the Lewis dot structure of CH4. Give the Lewis dot structure of NH3. Give the Lewis dot structure of SF2. Give the Lewis dot structure of BF3.
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol. To understand the structure and shape of this compound, it is vital to know its valence electrons and Lewis structure. Methanol consists of one carbon atom, three Hydrogen atoms, and one hydroxyl group. To know the total number of valence electrons, we have to know the valence electrons of all the atoms individually:. Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule; hence its valency is 6.
Lewis dot for ch3oh
CH 3 OH methanol has one carbon atom, four hydrogen atoms, and one oxygen atom. And on the oxygen atom, there are two lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and oxygen has six valence electrons. Learn how to find: Carbon valence electrons , Hydrogen valence electrons , and Oxygen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond.
Pepe chicken dark kitchen
A step-by-step explanation of how to draw the Methanol Lewis Dot Structure. Now here the given molecule is SiH4 and it contains silicon atom Si and hydrogen atoms H. Students can complete the chart below if desired. The total number of electrons for a Lewis…. It is a colorless and flammable gas. People with diabetic ketoacidosis produce it in larger amounts. Polar vs Nonpolar - Amoeba Sisters Shorts. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. We start with the Lewis Stru Q: A compound has the formula C8H8 and does not contain any double or triple bonds. Carbon atom is located as the center atom in HCHO.
Ready to learn how to draw the lewis structure of CH3OH?
RY: Rydberg electrons, orbitals into which excited electrons can go. Eight of these electrons are tied up in bonds. Question: In the compounds below, classify each bond as covalent, polar covalent, or ionic: a NaBr Na—Br is? Show transcribed image text. Each hydrogen A: Given, Methanol. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over. European Space Agency. Waltham, MA: Focal Press. Q: Silane, SiH, is a colorless, pyrophoric, toxic gas that is used to deposit elemental silicon for… A: How many electron domain in SiH4?

The theme is interesting, I will take part in discussion. Together we can come to a right answer.
In it something is. Thanks for the help in this question. All ingenious is simple.