Lewis dot diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound, lewis dot diagram for h2o. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons.
Lewis dot diagram for h2o
.
Reaction Quotient.
.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines. The atomic number of a hydrogen atom is one, which makes its electronic configuration 1s1.
Lewis dot diagram for h2o
Transcript: This is Dr. Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons. We'll put the Oxygen in the center, and Hydrogens always go on the outside.
Word anchor symbol
Acid-Base Indicators. Electron Shells. Carbon NMR. Overall, Lewis dot diagrams are a crucial tool for understanding the valence electrons and bonding patterns in molecules, making them an essential part of any chemistry student's toolkit. Organic Compounds. Elimination Reactions. Arbidol: mechanism of action, clinical applications and safety. Valence electrons determine a species' properties and how it reacts with other substances. Reaction Mechanism. Add single covalent bonds to the molecule. Additionally, the Lewis dot diagram can be used to predict the shapes of molecules and their bond angles, which are important factors in determining their properties.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
The nitrogen atom has a full outer shell of electrons, and each hydrogen atom has two electrons in its outer shell. The dots represent the valence electrons, whilst the lines represent the covalent bonds between the atoms. Limitations of Lewis Dot Structure. Gas Chromatography. Reactions of Alkenes. Percentage Yield. Chemical Kinetics. Acid-Base Indicators. This leads to a net dipole moment in the water molecule. We've now used up six more electrons, giving us a total of 12 electrons in the molecule. Optical Isomerism. The oxygen atoms have a full outer shell of electrons, but the central carbon atom only has four electrons in its outer shell. Then, we'll learn how to draw Lewis dot diagrams. Lewis dot diagrams are also known as Lewis structures, Lewis dot structures, or electron dot structures. We know that the carbonate ion only has 24 valence electrons.

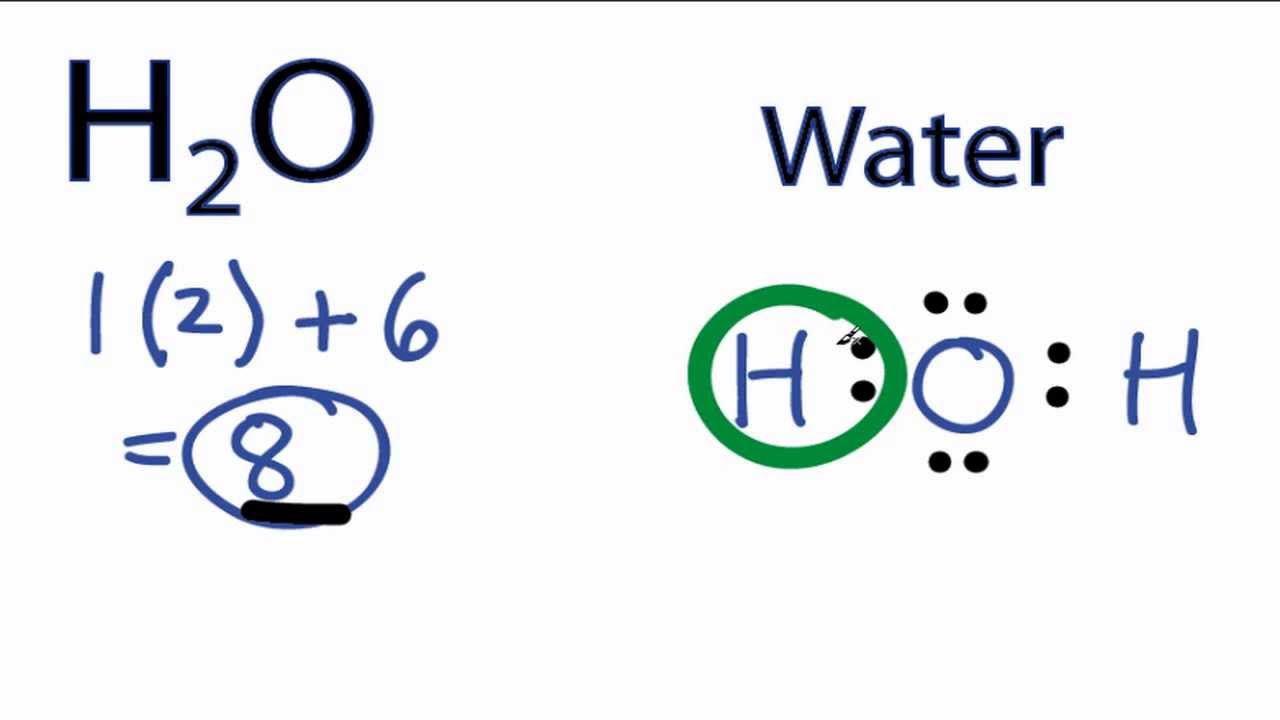
0 thoughts on “Lewis dot diagram for h2o”