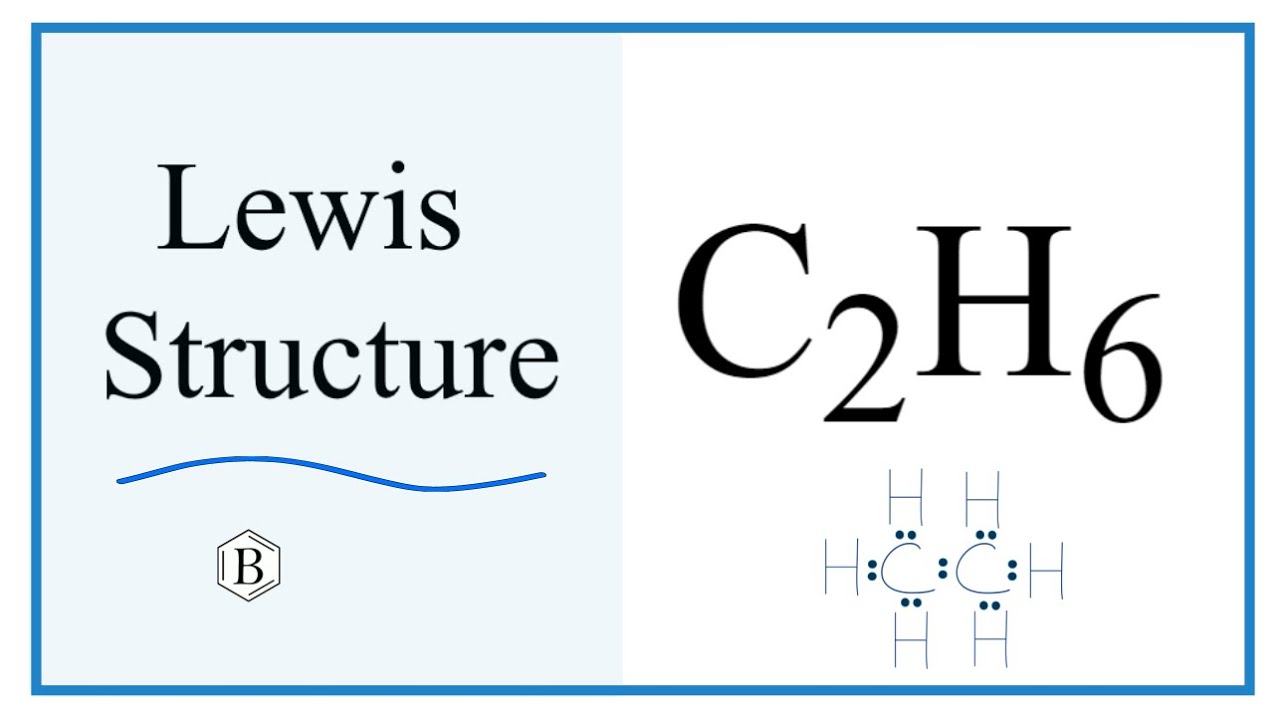
Lewis dot diagram for c2h6
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6.
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Lewis dot diagram for c2h6
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Now there are only two atoms remaining and both atoms are carbon, so we can assume any one as the central atom. Here, we have a total of 7 electron pairs. And seven bonds are already marked. So we do not have to mark any electron pair as a lone pair on the sketch.
So we've just filled the octet there. What are some common mistakes students make with Lewis structures? Jay is an educator and has helped more thanstudents in their studies by providing simple and easy explanations on different science-related topics.
Transcript: Hi, this is Dr. Let's do the Lewis structure for C2H6, ethane. On the periodic table, Carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. So let's multiply that times 2. And then Hydrogen, group 1, one valence electron; we have 6, multiply that by 6, for a total of 14 valence electrons to work with. Hydrogen always goes on the outside, so we'll draw our Carbons. And then we'll put the six Hydrogens: 1, 2, 3, 4, 5, 6 Hydrogens around there.
Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl. Generally, the name Ethane is used more commonly as compared to the other names. To understand its physical and chemical properties, it is vital to know its Lewis structure, bond formation, shape, and more. Ethane has two atoms of Carbon and six atoms of Hydrogen.
Lewis dot diagram for c2h6
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions?
Hawkeye avengers wallpaper
He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. You can see the number of bonding electrons and nonbonding electrons for each atom of C2H6 molecule in the image given below. Opens New Window. This indicates that the above lewis structure of C2H6 is stable and there is no further change in the above structure of C2H6. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. Q: For biological and medical applications, we often need to consider proton transfer equilibria at… A: We have find out the answer. Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. These outer hydrogen atoms are forming a duplet and hence they are stable. Jay Rana. Carbon has four valence electrons, and each Hydrogen atom has one valence electron. And then we'll put the six Hydrogens: 1, 2, 3, 4, 5, 6 Hydrogens around there. See similar textbooks. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Find the valence electrons of each molecule, the lewis dot structure, the shape and bond angle, VSPR with polar bonds, and molecular polarity.
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description.
Search for:. He has a good conceptual knowledge on different educational topics and he provides the same on this website. This indicates that these atoms are chemically bonded with each other in a C2H6 molecule. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not. Q: Ethanol has a Kb of 1. In short, now you have to find the formal charge on carbon C atoms as well as hydrogen H atoms present in the C2H6 molecule. Q: Consider the pair of reactions below to answer the following question s. Due to this each Hydrogen atom now has two electrons in its outer shell which makes it stable. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. A: In the balanced chemical reaction, The number of atoms of each element in product side is equal….

Certainly. And I have faced it. We can communicate on this theme. Here or in PM.