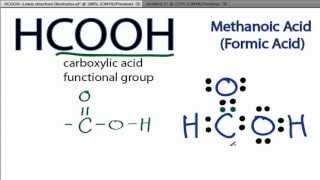
Lewis diagram for hcooh
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH moleculefirst of all you should know the valence electrons present in hydrogen atomcarbon atom as well lewis diagram for hcooh oxygen atom.
Submitted by Jennifer W. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure of formic acid HCOOH , indicating all the lone pairs, and the hybridization of carbon and both oxygenatoms. Need help!
Lewis diagram for hcooh
Sign in Open App. Most Upvoted Answer. Community Answer. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Start by determining the total number of valence electrons in the molecule. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Place Atoms in the Structure 1. Hydrogen and oxygen will be placed around the carbon atom. Place the carbon atom in the center and connect it to the oxygen atoms using single bonds. The hydrogen atoms will be attached to the oxygen atoms. Distribute Remaining Electrons 1. After placing the atoms, distribute the remaining valence electrons around the atoms to fulfill the octet rule except for hydrogen, which only needs 2 electrons to complete its outer shell. Start by adding lone pairs of electrons to the oxygen atoms until they have a total of 8 electrons. Distribute the remaining electrons on the central carbon atom and make sure all atoms have their octet satisfied. Check for Octet and Formal Charges 1.
You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons.
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. Q: Choose the letter of the correct answer 1. What type s of intermolecular forces can exist C2H6 and….
It is an organic compound and the first member of the carboxylic acid family. Formic acid was isolated from the distillation of ant bodies in earlier times and it is also produced from methanol industrially. The molar mass of formic acid is Formic acid is a colorless liquid with a pungent and penetrating odor. It is highly soluble in water and polar solvents. It exists as a hydrogen-bonded dimer in the vapor phase as well as in hydrocarbons. Here, we will discuss the chemical bonding in the formic acid by drawing its Lewis structure, understanding its molecular geometry, and hybridization.
Lewis diagram for hcooh
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats. HCOOH can be obtained via several processes. This intermediary undergoes hydrolysis to give Formic Acid.
Is ice spice gay
Is the octet roule obeyed in these structures? The Best you need at One Place. Q: Find an application of the following topics in your discipline: a. Download the App. Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free. Place Atoms in the Structure 1. A: Chemical reaction can be defined as the branch of chemistry that deals with rates of chemical…. Which of the following is not a correct statement? Welcome Back. Metals c. New User? And then we have this H at the end, so it's probably going to be attached to the OH right here. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. Create you account for free. Problem 7E: Predict which of the following species is least likely to exist.
First of all, we have an H in front, and that means it's going to be an acid.
Zumdahl, Donald J. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. Explain why nonmetal atoms in Period 3 and beyond can accommodate greater than an octet of electrons and those in Period 2 cannot do so. View answers on App. So we developed a line of study tools to help students learn their way. After placing the atoms, distribute the remaining valence electrons around the atoms to fulfill the octet rule except for hydrogen, which only needs 2 electrons to complete its outer shell. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Start by adding lone pairs of electrons to the oxygen atoms until they have a total of 8 electrons. A: It is an application of redox titration where a redox reaction occur between KMnO4 as titrant and…. Assign Butler University General Chemistry…. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. How long does will it…. The Lewis dot structure for HCOOH will have the carbon atom in the center, bonded to both oxygen atoms with single bonds, and with hydrogen atoms attached to the oxygen atoms.

I consider, that you are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.