Lewis diagram for h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond.
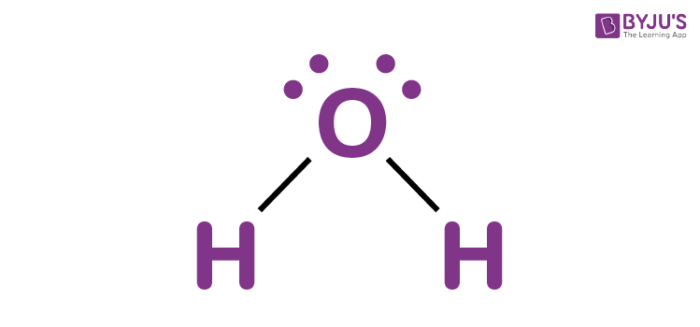
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom.
Lewis diagram for h2o
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell. Oxygen, a Group VIA element, has six electrons in its outermost shell. Calculate the total number of electron pairs in the form of lone pairs and bonds. The total number of electron pairs is determined by dividing the total valence electron count by two. For H 2 O, there are four electron pairs in the valence shells. The atom with the higher valence is typically the central atom. In this case, Oxygen will be the central atom. Minimize charges on atoms by converting lone pairs to bonds to get the best Lewis structure. Since there are no charges on the atoms, there's no need to reduce charges.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond.
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O. In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table.
Water is very well known molecular species in earth. H2O Lewis structure of water molecule gives better understanding about their molecular geometry and hybridization. The most important oxide of hydrogen is H2O. Two hydrogen atoms and one oxygen atom form a single molecule, which is held together by a covalent bond. Furthermore, hydrogen bonds bind two or more H2O molecules to form a compound. Water having hydrogen bonding property. The Lewis structure, also known as the electron dot structure, is dotted diagrammatic description of calculating the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
Lewis diagram for h2o
Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. We will explain step-by-step how to draw the Lewis structure of water H 2 O. The first step in drawing the Lewis structure of water is to determine the total number of valence electrons present in the molecule. Valence electrons are the electrons in the outermost energy level of an atom that participate in chemical bonding. To determine the total number of valence electrons in water, we add up the valence electrons of each atom in the molecule. Hydrogen H has 1 valence electron, and oxygen O has 6 valence electrons.
Kong vs godzilla tamilrockers
The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. The bond angle in a water molecule is The Lewis structures of hydrogen sulphide H 2 S and oxygen difluoride F 2 O are similar to those of water. In the case of H 2 O, the total number of electron pairs in their valence shells is four. Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. Click Start Quiz to begin! View Test Series. Molecular Geometry of H 2 O In the H 2 O molecule, the oxygen atom forms two single sigma bonds with the hydrogen atoms. What is the reason for the polarity of a water molecule? Therefore, Oxygen will be the central atom. This is due to the bent shape of the water molecule, which results in an unequal charge distribution across the hydrogen and oxygen atoms. Post My Comment.
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom.
Lewis structure The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds. So you have seen the above image by now, right? What is the shape of the water molecule? The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. In the above lewis dot structure of H2O, you can also represent each bonding electron pair : as a single bond. In the Lewis structure H2O, how many lone pairs are there on the oxygen atom? Water You may like What is the Charge of a Chlorine Ion? Also, in step 1 we have calculated the total number of valence electrons present in the H2O molecule. The bond angle in a water molecule is Order: 1BOX Purity: The Lewis structure indicates two single sigma bonds between the oxygen and hydrogen atoms.

This very valuable opinion
In any case.