Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is. Q: Q2.
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Nitrogen is a group 15 element on the periodic table.
Lewis diagram for ch3nh2
.
Expert Solution. A: Lewis structures also known as Lewis dot structures are diagrams that represent the valence….
.
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair. Methylamine is an organic molecule that is colorless in the gaseous state and is a derivative of ammonia. Moreover, this molecule has a strong pungent fishy smell and is used commercially to produce ephedrine, carbofuran, metham sodium, methyl formamide, theophylline, carbaryl, N-methyl pyrrolidone. Methylamine is a known nucleophile where it bonds with the electrophiles by donating the electron pairs, which makes it a Lewis base. A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration. The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation. To study a chemical compound, the Lewis structure is the first step, to begin with as it helps with determining from which group the molecule belongs in alignment with the periodic table. It is a property conducive to covalent compounds mainly and is used to study formal charge on the participating atoms and chemical bonding taking place between the atoms.
Lewis diagram for ch3nh2
Ready to learn how to draw the lewis structure of CH3NH2? The Nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3NH2. Here, the given molecule is CH3NH2. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Nitrogen is a group 15 element on the periodic table.
The golden lion winchester menu
Author: David W. Now you have come to the final step in which you have to check the stability of lewis structure of CH3NH2. Principles of Modern Chemistry 8th Edition. Jay Rana. Such a structure is unusual…. A: Lewis dot structure represents the valence electron that is present in the atom or molecule. Q: Sketch BeH2 showing the orbitals and any overlapping orbitals to indicate covalent bonds. Pat Gillis, Laurie J. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Show FC…. What is HCH bond angle implied by this drawing if you assume it is flat?
CH 3 NH 2 methylamine has one carbon atom, five hydrogen atoms, and one nitrogen atom. The carbon atom is attached with three hydrogen atoms, and the nitrogen atom is attached with two hydrogen atoms.
He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Draw the best Lewis Structure for both molecules in the space below: b. Polarity Of Water In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Let me explain the above image in short. Problem 39P: Assign formal charges to all atoms in the following Lewis diagrams. Q: Sketch BeH2 showing the orbitals and any overlapping orbitals to indicate covalent bonds. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom? The structure only shows the toms and hOn they are…. Q: hat would be the formal charge on Se and S and both Os for the most stable resonance structures of…. Which atom in this structure….

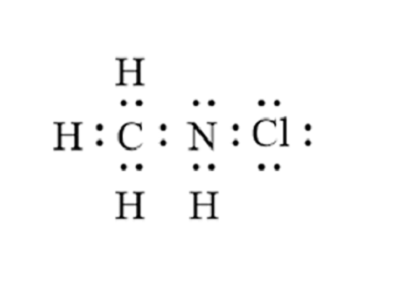
0 thoughts on “Lewis diagram for ch3nh2”