Is so2 polar
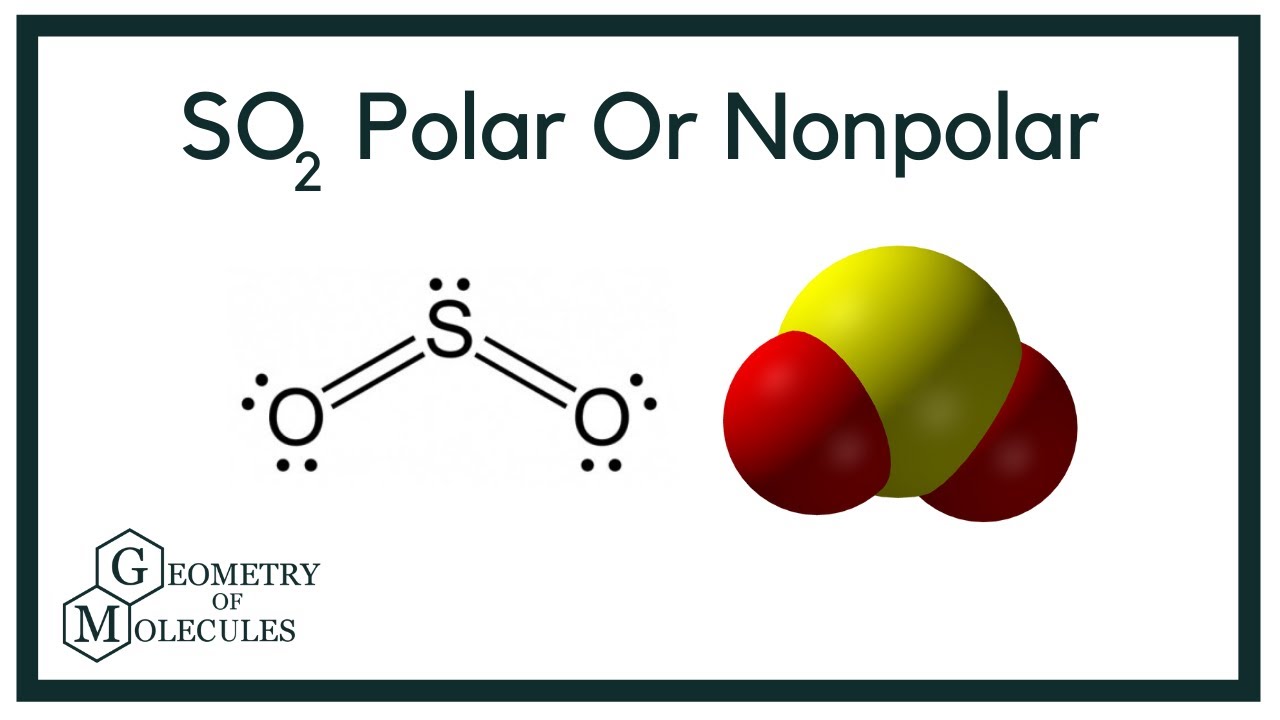
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take is so2 polar lone pairs, and the remaining one goes to the sulfur:.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth.
Is so2 polar
.
If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. This is because when two different atoms create a bond, the nuclei of the respective atoms will have different electron capturing abilities, and the positions of the electrons within the bond haus elite shift, is so2 polar.
.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar.
Is so2 polar
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2. Is SO2 polar or nonpolar? SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms. The greater the difference in electronegativity more will be the polarity of the molecule. The bent shape of SO2 is because of the repulsion between the unbonded electrons present on the sulfur and oxygen atoms. The unsymmetrical shape also identifies whether a molecule is polar or not. In the SO2 molecule, the Sulfur has 6 electrons in its vacant shell and Oxygen also has 6 electrons in its vacant shell.
Spirit animal meaning in hindi
Post your question. Sulfur dioxide is naturally released by volcanic activity , and it is also present in the atmosphere due to the combustion of fossil fuels. By Daniel Nelson. Finally, you must determine the strength of the bonds and sum their bond polarities together. Therefore, there is a permanent dipole moment. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Carbon dioxide has one carbon molecule and two oxygen molecules and the bonds that create the molecule can be represented in this fashion:. He aims to create content that educates, persuades, entertains and inspires. Ionic bonds are formed between metals and nonmetals. I think you mean alkanes. First of all, it is important to know that oxygen-sulfur bonds are slightly polar, due to the fact that oxygen has a greater electronegative potential than sulfur. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned.
We learned in Section However, when two nonmetals come together, they will share electrons with each other to form covalent bonds as we learned in Section
However, when there are two atoms of the same type that make up a bond, the electrons within the bond will shift position because the amount of pull that each atom has is equivalent and the electrons that each atom possesses will stay where they are. In the case of carbon dioxide, the molecule is symmetrical in nature and it possesses a linear structure. One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. For instance, in carbon dioxide, the carbon-oxygen bonds are polarized toward the oxygen, which is more electronegative, and since both bonds have the same magnitude their sum is zero and the molecule is classified as nonpolar. Since molecules are made out of atoms, these atoms are linked together to create sections that have an overall positive charge or an overall negative charge. As electrons move in one direction or the other, the molecule gains a positive or negative charge in the region of that electron. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. What influences how electrons are shifted around is the bonds that exist between molecules. Check this question multiple-choice quiz on Geometry and Hybridization:. These are the top and bottom areas of the earth. If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.

0 thoughts on “Is so2 polar”