Is pf3 polar or nonpolar
Post by ishaa Diwakar 4E » Thu Nov 14, am. Post by Jacob Motawakel » Sun Nov 17, am. Post by stephaniekim2K » Sun Nov 17, am.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Is pf3 polar or nonpolar
Q: Which of the following sets of atoms will NOT form a strong hydrogen bond? Explain your answer a. A: Hydrogen bonding: The hydrogen atom attached with the electronegative atom like fluorine, oxygen, or…. A: The molecule that shows dipole moment is known as polar molecule and bond is known as polar bond. Q: Is HCN polar. A: Definition of polar, Any compound or molecule is polar or not, it depends on the electronegativity…. Q: Which will have the lowest Bp? A: Boiling Point: The temperature at which the pressure exerted by the surroundings upon a liquid is…. Q: Which of the following is a hydrogen-bonding interaction circle one? Determine the polarity of the molecules. Methyl Chloride b.
Related questions.
Wiki User. PH3 is a non-polar covalent molecule. This is somehow confusing because, when you draw out the Lewis diagram, you will observe a lone pair on the P atom. However, if the electronegativity difference does not have a polar bond, then no matter what happens, it will always be non polar. In this case, the EN is 0.
PF3 is an odorless and colorless chemical compound. It has slow hydrolysis compared to other chemical compounds. Some may still be confused if PF3 is polar or nonpolar. So today, we will cover a topic on the polarity of this molecule. Molecules can either be polar or nonpolar, depending on the characteristics of the chemical compound.
Is pf3 polar or nonpolar
Phosphorus trifluoride is a chemical compound with the chemical formula of PF3. PF3 is known as a ligand in metal complexes, and it has a high level of toxicity in nature. To answer your question if Phosphorus Trifluoride is a polar or nonpolar molecule, our team did thorough research to provide you with all the information about the polarity of this compound. PF3 is a polar molecule. Due to the one lone pair present in the central atom Phosphorus, it generates repulsion. Nonpolar molecules have zero dipole moment, while polar molecules have greater than zero dipole moments. PF3 creates a dipole moment of 1.
Zomato momos price
KOH b. Masterton, Cecile N. Chemical Kinetics 2h 43m. Ester Reactions: Saponification. Magnetic Properties of Complex Ions. The molecule PF3 is polar. Skip to main content. Chemical Bonds. Solubility Rules. Simple Cubic Unit Cell. Transition Metals and Coordination Compounds 3h 14m. Titrations: Weak Base-Strong Acid. Quantum Numbers: Nodes. Power and Root Functions -.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
A: Since you have asked multiple questions, we will solve the first question for you. Organic Chemistry: A Guided Inquiry. CBr4 and 2. Answer the previous two questions for the antibonding molecular orbital in HF. ISBN: Hess's Law. A: The difference in electronegativity between fluorine and selenium is 4. Rutherford Gold Foil Experiment. Naming Benzene. Law of Multiple Proportions. Molecular Orbital Theory.

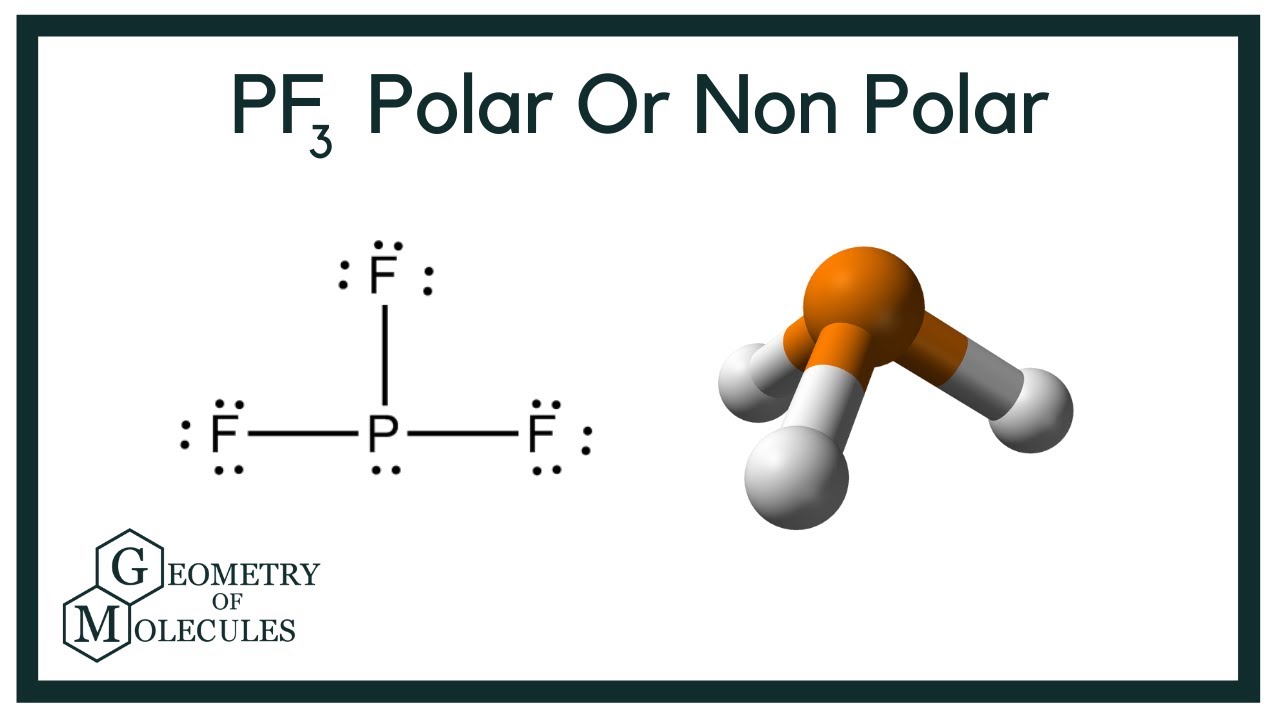
I think, that you are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.