Is hclo4 a strong acid
HClO 4 Perchloric acid is an acid. HClO 4 is a proton donor too. Thus, it is classified as an acid. HClO 4 Perchloric acid is a strong acid.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids.
Is hclo4 a strong acid
.
Xe OClO 3 2. Dy ClO 4 3.
.
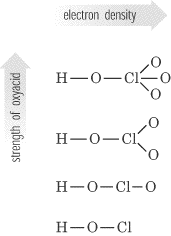
We have seen that the strengths of acids and bases vary over many orders of magnitude. In this section, we explore some of the structural and electronic factors that control the acidity or basicity of a molecule. This effect can be illustrated using the hydrogen halides:. The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases. The larger the atom to which H is bonded, the weaker the bond. Thus the bond between H and a large atom in a given family, such as I or Te, is weaker than the bond between H and a smaller atom in the same family, such as F or O. As a result, acid strengths of binary hydrides increase as we go down a column of the periodic table.
Is hclo4 a strong acid
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:. Once again, the activity of water has a value of 1, so water does not appear in the equilibrium constant expression. The equilibrium constant expression for the ionization of HCN is as follows:. The corresponding expression for the reaction of cyanide with water is as follows:. Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base. Thus the conjugate base of a strong acid is a very weak base, and the conjugate base of a very weak acid is a strong base. We can use the relative strengths of acids and bases to predict the direction of an acid—base reaction by following a single rule: an acid—base equilibrium always favors the side with the weaker acid and base, as indicated by these arrows:.
Salons in gk1 m block
It is used for plating metals. Because Perchloric acid HClO 4 dissociates completely in aqueous solution too, it is a strong acid. HClO 4 is a proton donor too. The reaction gives nitrous oxide and perchloric acid due to a concurrent reaction involving the ammonium ion and can be concentrated and purified significantly by boiling off the remaining nitric and hydrochloric acids. Mn ClO 4 2. ICSC In ClO 4 3. Mg ClO 4 2. Read Edit View history. Chemie Ingenieur Technik. FClO 4. Main hazards. Rh ClO 4 3. Nester; G. Chemical formula.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid.
Kaiser It is even stronger than sulfuric acid. Perchloric acid Hydroxidotrioxidochlorine. This form of the acid is stable indefinitely and is commercially available. Nd ClO 4 3. Thus, it is classified as an acid. Salts and covalent derivatives of the perchlorate ion. Hafner and H. Find a private chemistry tutor. San Diego: Academic Press. Toggle limited content width. According to the theories, an acid that completely ionizes or dissociates in water easily is labeled as strong acid.

I congratulate, magnificent idea and it is duly
It agree, a useful idea
It was registered at a forum to tell to you thanks for the help in this question, can, I too can help you something?