Is diamond good conductor of electricity
There are two types of conductivity.
Computer simulations show diamonds can be made to conduct electricity like metal, and the potential real-world applications are numerous. An international team of scientists has discovered that diamonds can conduct electricity when put under strain at a nanoscopic scale. While diamond naturally acts as an electrical insulator, it can be made to carry electrical current when in the form of nanoscopic needles. When a diamond nano-needle is put under strain or bent , its properties begin to change, allowing it to conduct electricity much like a conductive metal. An electrical conductor is any material capable of carrying electrical current.
Is diamond good conductor of electricity
.
Thermal conductivity is a measure of how well a material conducts heat.
.
Laurens H. Willems van Beveren does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment. Diamond is well known for its appeal as a gemstone. Perhaps less well known are some of its extreme material properties. As well as being the hardest material in nature, diamond is very good at conducting heat at elevated temperatures making it an ideal material for heat-management applications. My colleagues and I at the University of Melbourne and La Trobe University are harnessing some of these less-well-known material properties of diamond in order to, hopefully, develop the next generation of transistors.
Is diamond good conductor of electricity
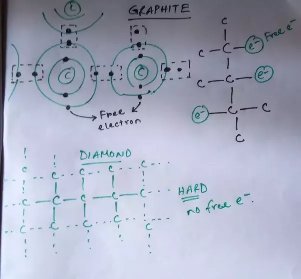
There are two types of conductivity. Thermal conductivity is a measure of how well a material conducts heat. Electrical conductivity expresses how well a substance conducts electricity. A diamond has characteristic thermal and electrical conductivity that can be used to help distinguish it from other materials and identify impurities in a genuine diamond. Most diamonds are extremely efficient thermal conductors, but electrical insulators. Diamond conducts heat well as a result of the strong covalent bonds between carbon atoms in a diamond crystal. The high thermal conductivity may be used to distinguish diamond from cubic zirconia and glass. Moissanite, a crystalline form of silicon carbide that resembles diamond, has a comparable thermal conductivity. Modern thermal probes can differentiate between diamond and moissanite, as moissanite has gained popularity.
Kami buffet & grill menu
Create profiles for personalised advertising. Measure advertising performance. Develop and improve services. Common examples include metals such as copper and aluminum, which are found in nearly all electrical wiring. The research team, which included researchers from the Skolkovo Institute of Science and Technology Skoltech , Russia, used computer simulations involving quantum mechanics to see the effects of bending and stretching diamond nano-needles. These choices will be signaled to our partners and will not affect browsing data. Metallic Bond: Definition, Properties, and Examples. Metallization of diamond. Diamond is also an insulator in its natural state, but this new discovery shows that diamonds are capable of providing the best of both worlds. Use profiles to select personalised advertising.
Diamond has one of the most unique sets of physical properties among all of the other elements due to which sometimes one can get confused over classifying its physical properties. So, does diamond conduct electricity? Diamond does not conduct electricity although it is a good thermal conductor.
These needles, which are roughly one thousand times thinner than a strand of human hair, were bent side to side by a large diamond probe. Most diamonds are extremely efficient thermal conductors, but electrical insulators. Sunlight Solution To Water Shortages. There are two types of conductivity. For a diamond, a bit of additional strain can often be electrifying. There are currently known minerals, from abellaite to zykaite. Skip to content New Technologies Physics. Diamond conducts heat well as a result of the strong covalent bonds between carbon atoms in a diamond crystal. When the strain is released, the diamonds simply snap back to their original insulator form. In light of this new research, perhaps we can add an amendment to that sentiment. The Chemistry and Structure of Diamonds. Synthetic diamonds doped with boron also are p-type semiconductors. More than just a pretty rock, it appears that diamonds may have finally found their most practical purpose. Common examples include metals such as copper and aluminum, which are found in nearly all electrical wiring.

Yes, I understand you.
The authoritative point of view, curiously..