Is chcl3 polar or nonpolar
Post a Comment. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms, is chcl3 polar or nonpolar. Since the one hydrogen on the structure 2.
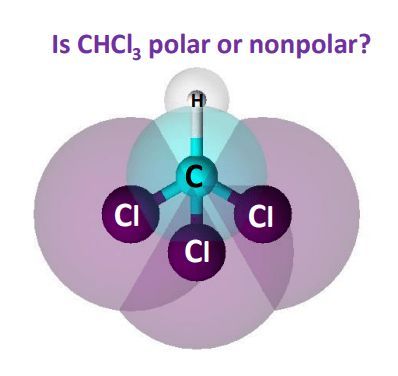
CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of its molecule. CHCl3 is polar due to its tetrahedral molecular structure and difference between the electronegativity of C, H and, CL. Chlorine atoms are more electronegative than carbon and hydrogen and lie at three vertices of the pyramid and pull the negative charge to its direction making it a polar molecule with a dipole in a downward direction. There are three certainties in this world: Death, Taxes and Homework Assignments. No matter where you study, and no matter….
Is chcl3 polar or nonpolar
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn't matter which direction the dipole moment vectors are pointing out or in. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. As mentioned in section 4. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. A molecule with two poles is called a dipole see figure below.
However, they need to be checked by the moderator before being published.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. About About this video Transcript. Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Molecular polarity depends on both individual bond polarities and molecular geometry, the latter of which we can predict using VSEPR theory.
Is chcl3 polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity , a scale for judging how much atoms of any element attract electrons. Electronegativity is a unitless number; the higher the number, the more an atom attracts electrons.
Tv schedule tv guide
CHCl3 is polar due to its tetrahedral molecular structure and difference between the electronegativity of C, H and, CL. No matter where you study, and no matter…. Post a Comment. The molecule is symmetric. Contributors and Attributions StackExchange thomij. Steps to Identify Polar Molecules Draw the Lewis structure Figure out the geometry using VSEPR theory Visualize or draw the geometry Find the net dipole moment you don't have to actually do calculations if you can visualize it If the net dipole moment is zero, it is non-polar. Learn more about our help with Assignments: Inorganic Chemistry. Numbers and figures are an essential part of our world, necessary for almost everything we do every day. Place free inquiry. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2.
It is also commonly known by the term chloroform. It exists as a colorless dense liquid having a sweet smell. Many of you might have doubts regarding whether CHCl3 is polar or not.
Be the first! In contrast, water is polar because the OH bond moments do not cancel out. Comments No comments. Contributors and Attributions StackExchange thomij. The enthalpy of vaporization of HF lower than C2. Inorganic Chemistry. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. The molecule is symmetric. Hydrogen fluoride is a dipole. Sign in. Draw the MO of the hypothetical species square H4 3. This means that the compound is a liquid at standard temperature and pressure. Our fields of expertise.

In my opinion you are not right. I can defend the position. Write to me in PM.
Be assured.
It is remarkable, very useful piece