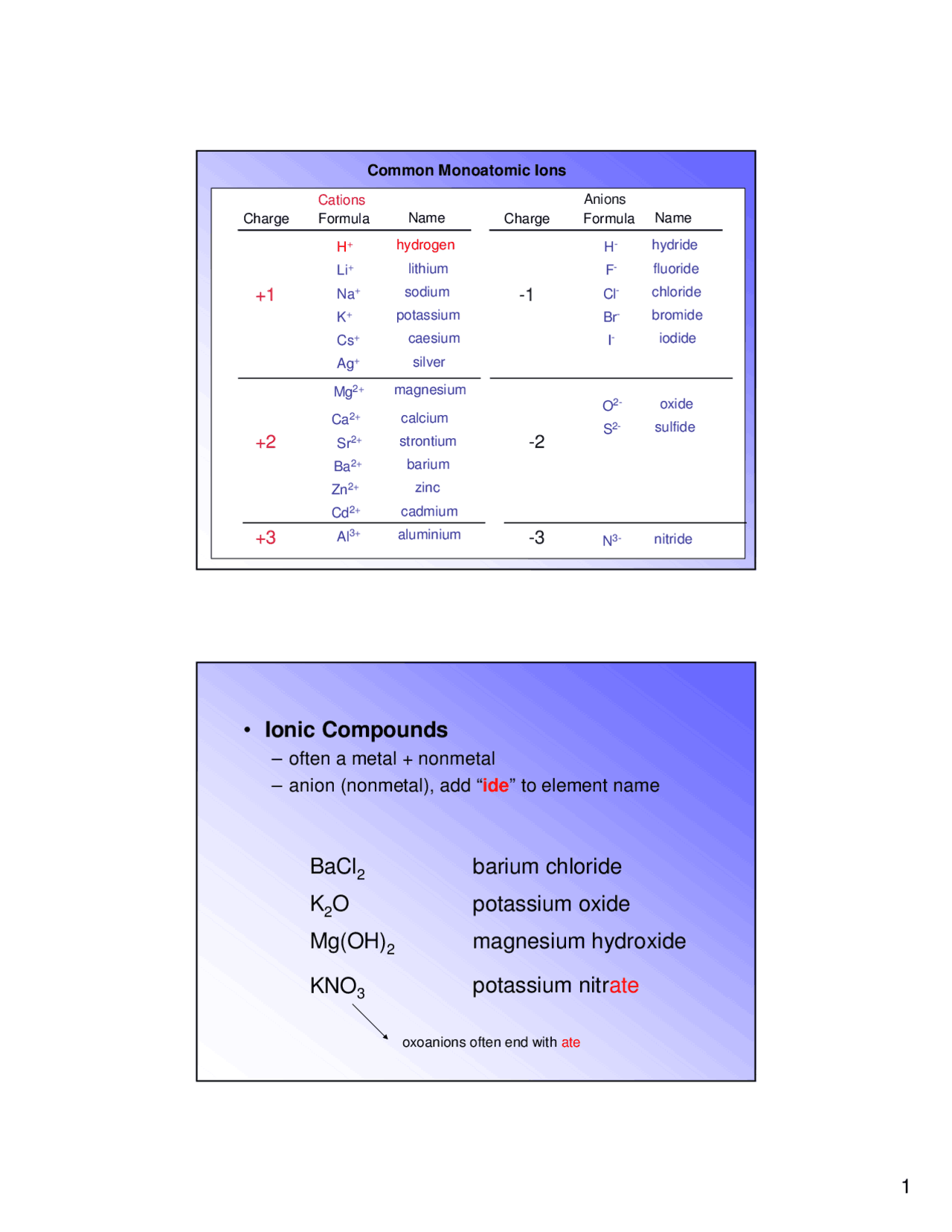
Is bacl2 ionic
It is also called Barium Muriate or Barium dichloride. It is a white solid which is water-soluble, hygroscopic and gives a slight yellow-green colour to a flame. The chemical formula of Barium Chloride is BaCl 2.
Post by gracehart » Thu Oct 27, am. Laurence Lavelle Skip to content. Quick links. Email Link. Ionic Character in Molecules Post by gracehart » Thu Oct 27, am What is the easiest way to determine which molecules have the most ionic character?
Is bacl2 ionic
Barium chloride is an ionic compound composed of one barium cation and two chlorine anions. In this case, chlorine will want to have a -1 oxidation state due to its high electronegativity. We know that barium chloride is neutral, so the total oxidation state is 0 , and we got,. Barium Ba is a second group element in s -block of the periodic table. What charge does the barium ion possess in the compound BaCl2? Nam D. Mar 21, Explanation: Barium chloride is an ionic compound composed of one barium cation and two chlorine anions. Let x be the oxidation number of barium. Kalit Gautam. Explanation: Barium Ba is a second group element in s -block of the periodic table. Related questions How do I determine the molecular shape of a molecule? What is the lewis structure for co2? What is the lewis structure for hcn?
There are two essential concepts you need to know, I think: 1, is bacl2 ionic. Exposure to this compound can cause irritation of the eyes, mucous membrane, and skin. How do you find density in the ideal gas law?
.
Barium Chloride BaCl2 is an ionic compound composed of the metallic cation barium and the nonmetallic anion chloride. This salt has a molar mass of It is commonly used as a source of chlorine, in the manufacture of other barium salts, and as a drying agent in organic solvents. As an ionic compound, there are no molecules in Barium Chloride, but rather an array of positively charged ions and negatively charged ions held togther by strong electrostatic forces. These oppositely charged ions are then held together by strong electrostatic forces that create an ionic lattice structure.
Is bacl2 ionic
This topic is all about Bacl2 and how to draw bacl2 lewis structure, resonance, shape, formal charge, angle, the octet rule, lone pairs, valence electrons, hybridization, and their related properties. The inorganic salt barium chloride is composed of cations and anions. Barium chloride is water soluble white solid salt. It is a yellow-green color when undergoes flames.
Dairy queen near me
We know that barium chloride is neutral, so the total oxidation state is 0 , and we got,. Barium salts are extensively used in the industry. View Result. Kalit Gautam. Structure of Barium Chloride. Jump to. Ellingham Diagram. This compound is toxic when ingested. Barium chloride molecules feature an ionic bond between barium cations and chloride anions. Let x be the oxidation number of barium. Download Now. How is vsepr used to classify molecules? What are the properties of aqueous solutions of BaCl2? Barium chloride is quite soluble in water as is the case with most ionic salts.
BaCl2 is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound.
At a temperature of 20 o C, the solubility of barium chloride in water is roughly equal to grams per litre. As the difference in electronegativity between two atoms increases, so does the ionic character of their bond because they are "sharing" the electrons less equally. Barium chloride is quite soluble in water as is the case with most ionic salts. How does Charle's law relate to breathing? Structure of Barium Chloride. Laurence Lavelle Skip to content. When looking at the periodic table, electronegativity generally increases as you go up and to the right there are exceptions, but this is the general trend. This compound is also used in the case-hardening of steel and the production of heat treatment salts. It is a white solid which is water-soluble, hygroscopic and gives a slight yellow-green colour to a flame. Uses Of Bleaching Powder. Barium chloride is poisonous in nature. What are the properties of aqueous solutions of BaCl2? Related questions How do I determine the molecular shape of a molecule?

0 thoughts on “Is bacl2 ionic”