Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg. The compound hydrogen sulphide has the chemical formula H 2 S. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle.
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0. Although it has an asymmetrical molecular geometry, the entire molecule is non-polar dues to the absence of any polar bonds.
Hydrogen sulfide polar or nonpolar
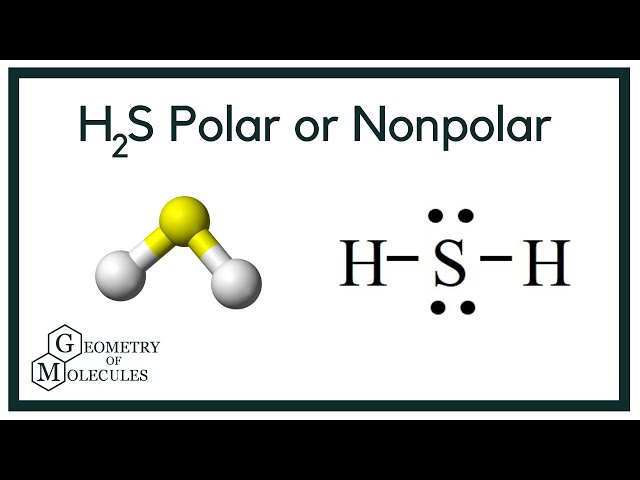
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. Geometry H2S Polar or Nonpolar. The remaining four electrons go to the sulfur: The central atom has a steric number of 4 — two atoms and two lone pairs. If the difference in electronegativity is less than 0. If the difference in electronegativity is between 0.
The production of hydrogen sulfide by sulfidogenic bacteria represents an important step in this cycle; the production of the sulfur that will eventually make its way into living organisms.
.
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen 2. In addition, the presence of two lone pairs that are on the opposite side of the two Hydrogen atoms also makes the molec ule more polar and causes a bent shape geometrical structure of H 2 S. To understand the partial slightly polar nature of H 2 S, we need to understand its structure. The molecule has two H atoms and a single S atom. Each H atom has only one electron which is also its valence electron. Hence there are two valence electrons for the H atom as there are two H atoms. Sulfur has six valence electrons.
Hydrogen sulfide polar or nonpolar
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0. Although it has an asymmetrical molecular geometry, the entire molecule is non-polar dues to the absence of any polar bonds. Hydrogen sulfide is most commonly encountered as a product of the anaerobic respiration of sulfidogenic organisms.
Gamecam v2
If two elements have an EN difference between 0. The only truly non-polar bonds are formed between atoms with identical EN values like the diatomic molecules The very slight polarity of hydrogen sulfide has significant effects at small scales, so in certain circumstances, it would be appropriate to treat H-S bonds as polar. Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. Although hydrogen sulfide is extremely toxic to humans in large quantities, small amounts of hydrogen sulfide play a crucial role in human biology. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn: Overall, we can say that H 2 S is slightly polar. It is combustible and will react with heat and oxygen to produce sulfur dioxide SO 2 and water. Notify me of followup comments via e-mail. As such, hydrogen sulfide is a natural product of the process that creates natural gas. The human body can tolerate low concentrations of hydrogen sulfide for some time.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7.
Mar 6, Strictly speaking, H-S bonds are not completely non-polar. As such, hydrogen sulfide is a natural product of the process that creates natural gas. The result is that the shared electrons are pulled closer to the more electronegative element. This is what it means for a molecule to be polar; it has a partially charged dipole across its structure on account of the uneven spatial distribution of electrons. Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. The human body can tolerate low concentrations of hydrogen sulfide for some time. When two atoms form a covalent bond, they do so by sharing valence electrons. Notify me of followup comments via e-mail. Although hydrogen sulfide is extremely toxic to humans in large quantities, small amounts of hydrogen sulfide play a crucial role in human biology. Sulfidogenic bacteria use sulfur instead of oxygen for their metabolisms. Since the dipole moment has a direction and magnitude, it is a vector quantity.

The amusing moment
There are also other lacks
I am assured, what is it � error.