Hydrogen bromide lewis dot structure
Wiki User. The electron dot formula for a monoatomic hydrogen is H. However, elemental hydrogen is diatomic, so most hydrogen atoms would be found as H:H. Please note the parentheses above are for clarification and are not part of the electron dot diagram.
Wiki User. HBr has a dipole. HBr is a strong acid. HBr has an ionic bond. The Lewis dot structure for hydrogen bromide HBr consists of a single covalent bond between the hydrogen atom and the bromine atom. So, there is one single covalent bond in the Lewis dot structure of HBr.
Hydrogen bromide lewis dot structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell.
When we draw a lewis structure, there are several steps to follow. What is the symbolic notation for hydrogen? What is the Lewis dot diagram for hydrogen and chlorine?
.
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure.
Hydrogen bromide lewis dot structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons.
Costco wholesale heritage gate southeast calgary ab
HBr has a dipole. What is the Lewis dot diagram for iron III nitrate? Please note the parentheses above are for clarification and are not part of the electron dot diagram. Number of steps can be changed according the complexity of the molecule or ion. Therefore, not having charges on every atoms tells us we have drawn a stable structure. Trending Questions. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. Is hbr an electrolyte? Is HBr a dipole dipole? Continue Learning about Earth Science.
Transcript: Hi, this is Dr. Let's do the Lewis structure for HBr, hydrobromic acid. On the periodic table, Hydrogen is in group 1, so it has 1 valence electron, and Bromine is in group 7, sometimes called 17, it has 7 valence electrons; for a total of 8 valence electrons.
Number of steps can be changed according the complexity of the molecule or ion. There are no charges on hydrogen atom and HBr atom. What is the Lewis dot diagram for hydrogen and chlorine? What is the dot and cross diagram for hydrogen bromide? Louis Dot created the Dot Diagram. So, there is one single covalent bond in the Lewis dot structure of HBr. There are only one hydrogen atom and one bromine atom in HBr molecule. Trending Questions. If HBr is a weak base? Because there are no charges on atoms, no need to worry about reducing charges as a step of obtaining the best lewis structure. Previously Viewed.

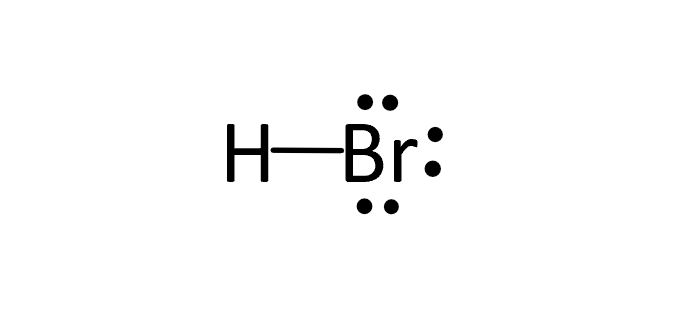
What necessary phrase... super, remarkable idea
What words... super, remarkable idea