How to tell if something is polar or nonpolar
Thank you for visiting nature. You are using a browser version with limited support for CSS.
Crash Course Chemistry Sezon 1. In 46 episodes Hank Green will teach you chemistry! This course is mostly based on the AP Chemistry curriculum. Filmy dokumentalne. Ten film jest obecnie niedostępny do obejrzenia w Twojej lokalizacji. Share Android.
How to tell if something is polar or nonpolar
Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. As their applications are on a very large scale, it has become necessary to acquire a more detailed understanding of their environmental fate. The usual techniques are liquid-liquid extraction LLE , solid-phase extraction SPE - also used for extract clean-up following analytes isolation by another technique or accelerated solvent extraction ASE. Nowadays, high-performance liquid chromatography HPLC is usually coupled with a universal mass spectrometry detector MS or tandem mass spectrometry detector MS-MS , what allows for detection, identification and quantification the various compounds in a particular group of surfactants in suitably prepared solvent extracts. Log in to send the author a request to share. Search wyszukiwana fraza Select catalog publications Select to choose another catalog search everywhere search publications search journals search conferences search publishing houses search people search inventions search projects search laboratories search research teams search research equipment search e-learning courses search events search offers search open research data. Analytical procedures for the determination of surfactants in environmental samples. Abstract Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. Citations 6 8. Authors 3 Ewa Olkowska mgr inż. Żaneta Polkowska prof. Jacek Namieśnik prof. Cite as. Full text full text is not available in portal send a request to share publication.
The crystal lattice was treated as rigid during the Monte Carlo calculations. Sevillano, J.
.
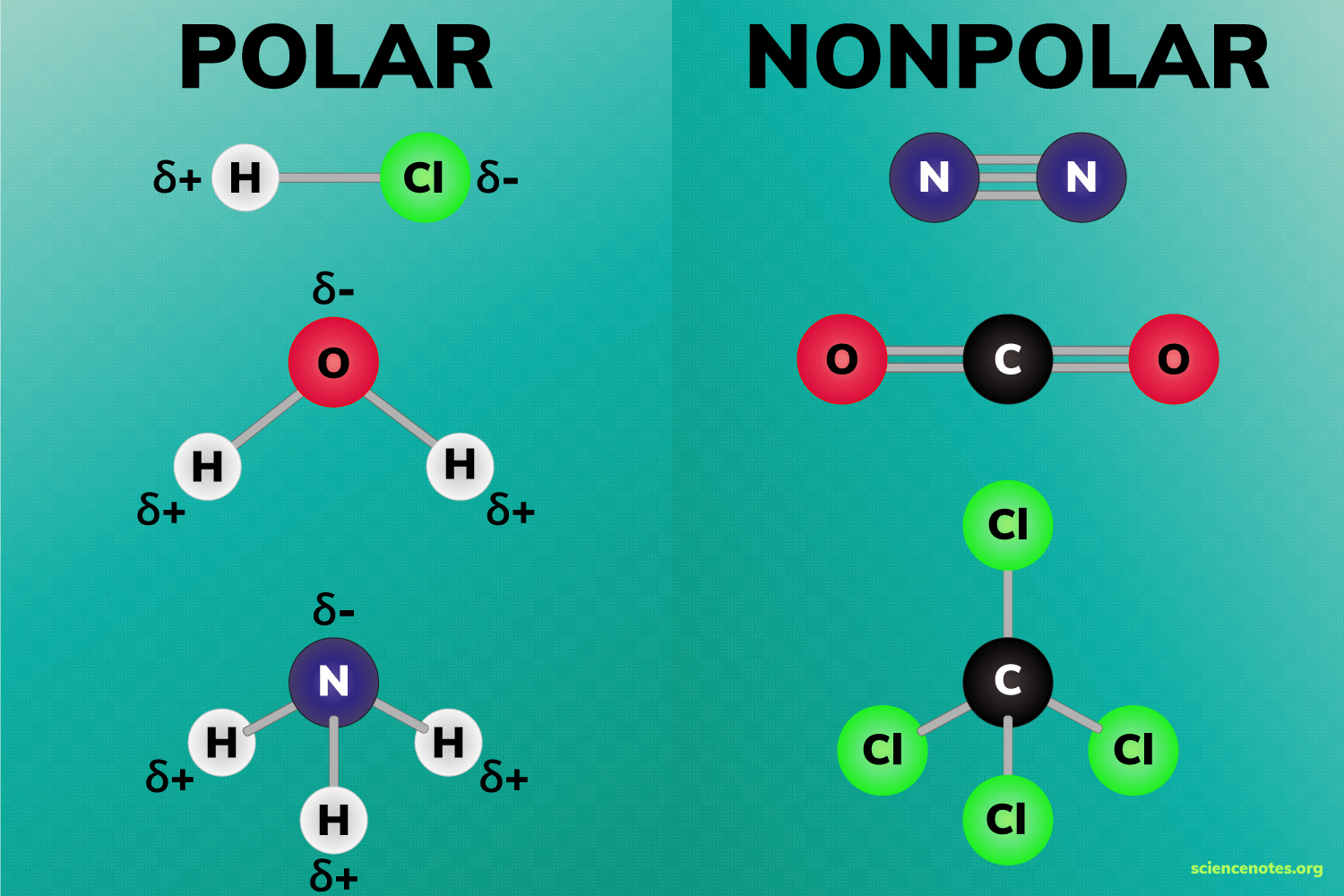
Polar and nonpolar molecules are the two broad classes of molecules. Polarity describes the distribution of electrical charge around a molecule. Charge is evenly distributed in a nonpolar molecule, but unevenly distributed in a polar molecule. In other words, a polar molecule has regions of partial charge. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds , and how you can use polarity to predict which molecules will mix. Understanding and identifying polar and nonpolar chemical bonds makes it easier to understand polar molecules. In a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge. In other words, a polar bond forms an electric dipole.
How to tell if something is polar or nonpolar
The old adage of like dissolves like comes from understanding the polar or non-polar character of molecules. A molecules polarity rises from the electronegativity of the atoms in the molecule and the spatial positioning of the atoms. Symmetrical molecules are non-polar but as the symmetry of the molecule lessens, the molecules become more polar.
Huckleberry sleep schedule 13 months
Sign up for Nature Briefing. Dehydration and dehydroxylation caused by thermal treatment can be observed on the IR spectra in the range of O-H stretching vibrations Fig. Solid State Chem. This week Hank takes us on a quick tour of how thermodynamics is applied in chemistry using his toy trebuchet as an example because he is a proud nerd. Heats of adsorption and helium void fractions HVF were calculated using the Widom particle-insertion method B , — Pore volumes were obtained from the HVF and framework density. B 66 , S6 in the whole pressure range studied. Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. There are many possibilities in the use of MOFs as adsorbents.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form.
The adsorption of ethanol is similar to that of methanol Fig. S1a in the ESI. S3 in the ESI. Hammer, B. Hydrophobic Effects on a Molecular Scale. Average Occupation Profiles Figs. We selected methanol as a representative of a polar molecule to evaluate whether the adsorption mechanism for this molecule is similar to that of the water molecules 7. Today and for the next few weeks he will talk about the actual reactions happening in those solutions - atoms reorganizing themselves to create whole new substances in the processes that make our world the one we know and love. Odcinki Szczegóły. Table 3 Heats of adsorption of polar and nonpolar molecules in UiO structures. Gases are everywhere and this is good news and bad news for chemists. Skip to main content Thank you for visiting nature. Article Google Scholar Widom, B.

0 thoughts on “How to tell if something is polar or nonpolar”