How to count sigma and pi bonds in benzene
Key Points.
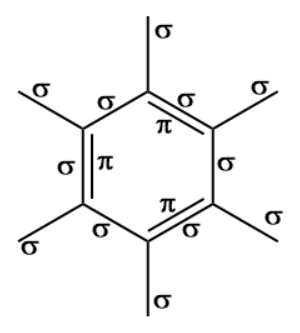
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. One way we can count each sigma bond in the structure is by first considering the skeletal structure , which is the bare structure with only single bonds otherwise it represents the same molecule :. From this, recall that one single bond contains one sigma bond. The sigma sigma bonds are simply the number of single bonds shown here:. Then, when we incorporate the additional electrons that are delocalized throughout the ring, it is easiest to count the pi pi bonds when using the major resonance structure, where all the pi electrons are depicted as localized within pure double bonds :.
How to count sigma and pi bonds in benzene
The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Earlier Badertscher et al. In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in a given unsaturated hydrocarbon containing double bonds. In this case, first we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated hydrocarbon containing double bonds. The total number of single bond for an aliphatic straight chain olefin is. Examples have been illustrated in Table 1. Straight-chain Structure. C H In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated cyclic olefinic hydrocarbons. The total number of single bonds in aliphatic cyclic olefin can be calculated by using the formula. Examples have been illustrated in Table 2. Single bonds A c. Cyclobuta diene.
Which gas is used for the preparation of soda water?
.
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar. As can be seen in the figure below, the electron domain geometry around each carbon independently is trigonal planar.
How to count sigma and pi bonds in benzene
This article builds on knowledge about the bonding in methane , and the bonding in ethene. You may also find it useful to read the article on orbitals if you aren't sure about simple orbital theory. You can also read about the evidence which leads to the structure described in this article. Benzene is built from hydrogen atoms 1s 1 and carbon atoms 1s 2 2s 2 2p x 1 2p y 1. Each carbon atom has to join to three other atoms one hydrogen and two carbons and doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s 2 pair into the empty 2p z orbital. There is only a small energy gap between the 2s and 2p orbitals, and an electron is promoted from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when these electrons are used for bonding more than compensates for the initial input. Because each carbon is only joining to three other atoms, when the carbon atoms hybridise their outer orbitals before forming bonds, they only need to hybridise three of the orbitals rather than all four.
Royalty house banquet facility warren mi
Related questions How does a molecular orbital differ from an atomic orbital? It is highly inflammable and very stable. Go back to previous article. Bonding in Organic Compounds. Which among the following is not a globally accepted National 'hot spot' of India? Straight-chain Structure. Molar mass: Sep 4, It is a natural component of crude oil and used as a solvent for fats, resins etc. The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Truong-Son N. Key Points. The homolytic fission of a covalent bond liberates :. In this case, first we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated hydrocarbon containing double bonds.
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:.
The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Heat is evolved during. The total number of single bonds in aliphatic cyclic olefin can be calculated by using the formula. Trusted by 5. This question was previously asked in. Testbook Edu Solutions Pvt. The candidates will be selected on the basis of their performance in prelims, mains, and personality tests. The chief ore of aluminium is. The aromatic nature of benzene is because of the continuous cyclic pi bond between the carbon atom. Impact of this question views around the world. More General Science Questions Q1. The total number of single bond for an aliphatic straight chain olefin is. International Agency for Research on Cancer IARC has classified radiofrequency electromagnetic fields as possibly carcinogenic to humans. Which German chemist used the technique of preparing hydrocarbons by electrolysis of solutions of salts of fatty acids?

You are not right. I am assured. I suggest it to discuss. Write to me in PM, we will talk.
Excuse for that I interfere � here recently. But this theme is very close to me. Is ready to help.
Completely I share your opinion. I think, what is it excellent idea.