How to convert grams to moles
This worked example problem shows how to convert the number of grams of a molecule to the number of moles of the molecule. This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Determine the number of moles of CO 2 in grams how to convert grams to moles CO 2. First, look up the atomic masses for carbon and oxygen from the periodic table.
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table.
How to convert grams to moles
With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. It can also work the other way around as a moles to grams conversion tool! Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future! Omni's mole calculator will help you gain knowledge. To correctly estimate the number of moles, n , of a substance of a specific mass, m , in grams , you need to follow the grams to moles formula:. But wait, what actually is a mole? The mole is the SI unit of measurement for the amount of a substance. In one mole of matter, there are precisely 6. This tremendous value refers to Avogadro's number feel free to check our Avogadro's number calculator to find more insights. In other words, it's the unit of quantity, similarly to a dozen or a gross. Anyway, that's quite a lot, isn't it?
If there is no subscript, it means there is only one atom of that element in the formula.
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources.
This worked example problem shows how to convert the number of grams of a molecule to the number of moles of the molecule. This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Determine the number of moles of CO 2 in grams of CO 2. First, look up the atomic masses for carbon and oxygen from the periodic table. The atomic mass of C is The formula mass of CO 2 is:. Thus, one mole of CO 2 weighs This relation provides a conversion factor to go from grams to moles. There are Sometimes you're given a value in moles and need to convert it to grams.
How to convert grams to moles
How heavy is 1. How many moles in Calculating the mass of a sample from the number of moles it contains is quite simple. We use the molar mass mass of one mole of the substance to convert between mass and moles.
Didactic synonym
Please log in with your username or email to continue. Yes No. With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. The molecular mass of NH 4 2 S is Cookies make wikiHow better. Do this for all the atoms and add the values to get the number of grams per mole. Use limited data to select advertising. This relation provides a conversion factor to go from grams to moles. This is your conversion factor. Mass of the substance, grams.
With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. It can also work the other way around as a moles to grams conversion tool! Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future!
You can always use our grams to moles calculator to check the result! Add the total weight of each element in the compound together. For example, NH 4 2 S has a molecular weight of 2 x The molar mass of KMnO4 is You will need the following: A pencil and paper. Thus, one mole of H 2 SO 4 weighs Farida Taha Oct 16, To convert from grams to moles, follow these few simple steps: Measure the mass of your sample in grams. Molar ratio This molar ratio calculator will help you determine the molar ratio between the different reactants and products in a balanced chemical reaction. Determine the number of atoms that each element contributes to the compound. Using a calculator, divide the number of grams by the molar mass. Not Helpful 17 Helpful Then, add all of your answers together to find the molar mass of the compound. Table of contents: How to calculate moles from grams? Create profiles for personalised advertising.

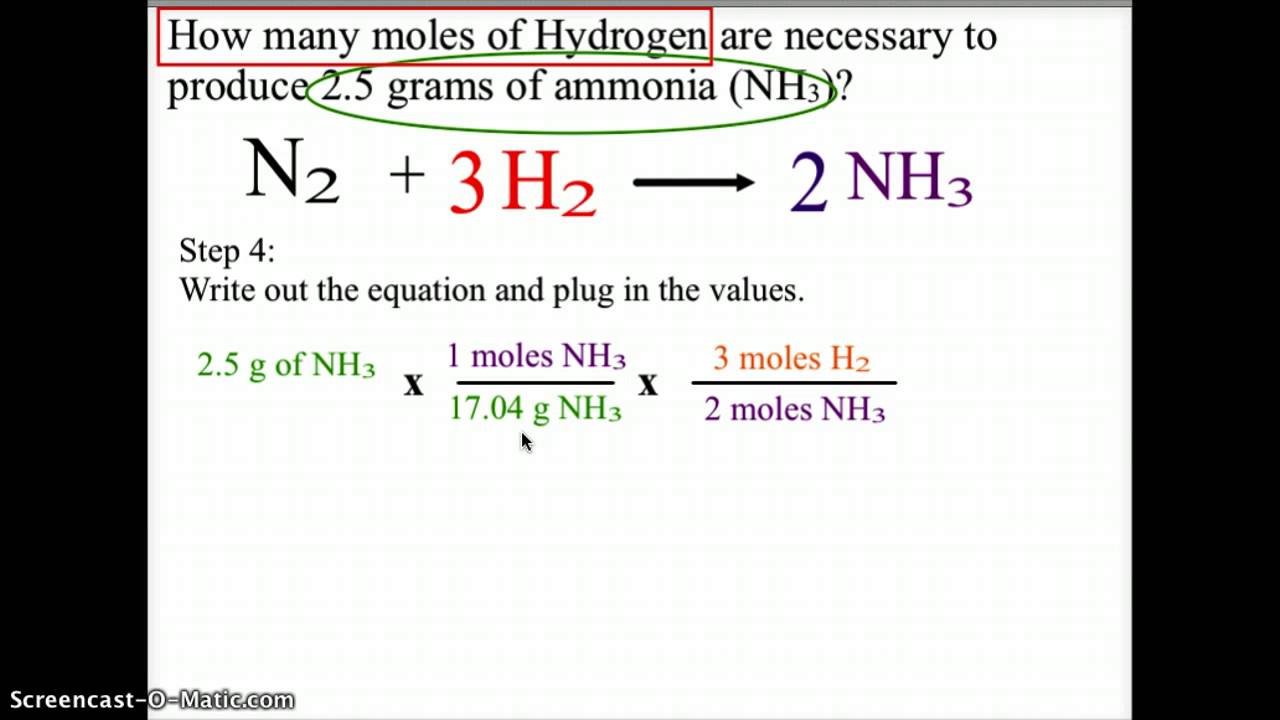
0 thoughts on “How to convert grams to moles”