How many valence electrons does n have
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. How many valence electrons does n have is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elementsthough. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have?
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table.
How many valence electrons does n have
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function. Molecular Orbitals. Sigma and Pi Bonds. Bonding Preferences. Formal Charges. Skeletal Structure.
Infrared Spectroscopy. Radical Reaction.
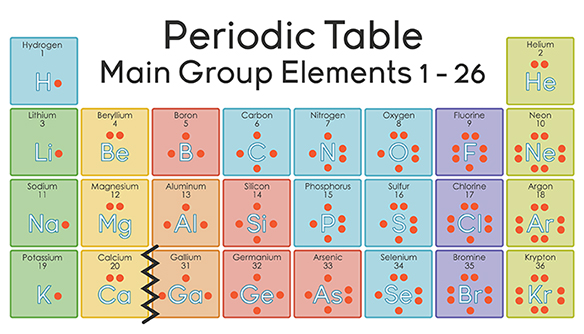
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple double or triple bonds to the central atom to achieve an octet. The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal. Each H atom group 1 has 1 valence electron, and the O atom group 16 has 6 valence electrons, for a total of 8 valence electrons. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
How many valence electrons does n have
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. Where you will get the HD images along with the explanation. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro. External links: Valence electrons of elements. Jay holds the roles of an author and editor at Periodic Table Guide, leveraging his ability to provide clear explanations on typically unexciting topics related to periodic table.
Feliz cumpleaños alejandra gif
Acid-Catalyzed Ester Hydrolysis. Reductive Amination. Enolate Chemistry:Reactions at the Alpha-Carbon 1h 53m. Conformational Isomers. Oxidation of Alcohols. Naming Amides. Stability of Conjugated Intermediates. Uses of nitrogen include anesthetic, refrigerant, and metal protector. NMR Spectroscopy. It is released from cars and is very toxic.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Cis vs Trans Conformations. How many valence electrons does oxygen have? Sinauer Associate. Beta-Dicarbonyl Synthesis Pathway. Organic Chemistry Reactions. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. Addition Reaction. Benzene Reactions. Almost all the oxides that form are gasses, and exist at 25 degrees Celsius. Naming Cycloalkanes. Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Monosaccharides - Strong Oxidation Aldaric Acid. Monosaccharides - Aldose-Ketose Rearrangement.

I congratulate, what words..., a remarkable idea
In my opinion you are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.
Bravo, what necessary words..., a remarkable idea