How many electrons are in chlorine
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science.
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloride , also known as table salt. Most people scientist know that the formula for salt is NaCl. One sodium Na atom gives it's electron to one chlorine Cl atom. Chlorine then has the eight electrons in its outer shell to make it "happy". Sodium is "happy" because it has now given up its one extra electron. Chem4Kids Sections.
How many electrons are in chlorine
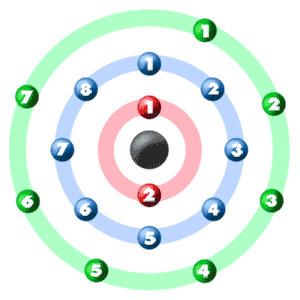
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons. Therefore, the number of electrons is equal to the number of protons in an chlorine atom. This means, that the number of electrons present in an chlorine atom is The electronic configuration of chlorine atom is:- E. Valence electrons are the number of electrons present in the outermost shell of an atom. Now, the last shell of chlorine atom has 7 electrons in it.
Chlorine has atomic number Video: Chlorine Electron Configuration Notation.
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital.
Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more. Elemental chlorine is commercially produced from brine by electrolysis, predominantly in the chlor-alkali process. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons.
How many electrons are in chlorine
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below.
Use abstruse in a sentence
How do valence electrons determine chemical properties? How many valence electrons are in an atom of bromine? Sodium chloride is the primary feedstock of chlorine for the chemical industry. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. The atomic number is the number of protons present in the nucleus of an atom. You can see in the diagram below that there are seven electrons in the outermost circle. Oct 27, Chlorine has 7 valence electrons. The electronic configuration of chlorine atom is:- E. Chlorine then has the eight electrons in its outer shell to make it "happy". Video from: Noel Pauller. Therefore, there are 7 valence electrons present in an chlorine atom. Therefore, the number of electrons is equal to the number of protons in an chlorine atom.
Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate. From the given table, for energy level 1, there's only 1 sublevel, which is called 1s.
This means, that the number of electrons present in an chlorine atom is Video: Chlorine Electron Configuration Notation. Magnesium is not only used to create alloys for manufacturing processes, but it is also the fourth most prevalent element in the human body and essential for nutrition. The name trichloride means three chlorine atoms are involved. The p orbital can hold up to six electrons. The next six electrons will go in the 2p orbital. Sodium chloride is the primary feedstock of chlorine for the chemical industry. Paper—Used as an oxidizing and bleaching agent in the pulp and paper industry. Sodium Chloride This is sodium chloride , also known as table salt. Magnesium chloride MgCl 2 —Found in seawater and serves as a natural source of metal magnesium. A workhorse element with a wide range of important applications Below are some of the primary uses of chlorine chemistry: Swimming pool water—Kills germs in pool water to help control the spread of waterborne illnesses. See all questions in Valence Electrons. How do valence electrons determine chemical properties? Impact of this question views around the world. Hydrochloric acid also has many uses including processing steel and food products like gelatin and sugar, and producing batteries.

0 thoughts on “How many electrons are in chlorine”