Hcl + koh reaction
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
Submitted by Joseph M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. If hydrochloric acid HCl reacts with the base lithium hydroxide LiOH , what are the products of the reaction?
Hcl + koh reaction
.
This is the most straightforward method. Photostudy and Studypool where I answered science and math problems. Balance the changes using electrons: Multiply the number of calcium atoms by 3 and the number of phosphorus atoms by 2.
.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients.
Hcl + koh reaction
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation in , so they are referred to as the Arrhenius definition of an acid and a base, respectively. Do we really have bare protons moving about in aqueous solution? The reaction of an acid and a base is called a neutralization reaction. In fact, the general reaction between an acid and a base is. In chemistry, the word salt refers to more than just table salt. By counting the number of atoms of each element, we find that only one water molecule is formed as a product.
Yuzu piracy
Log in to watch this video More Than Just We take learning seriously. A chemical equation represents a chemical reaction. If hydrochloric acid HCl reacts with the base lithium hydroxide LiOH , what are the products of the reaction? It shows the reactants substances that start a reaction and products substances formed by the reaction. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. Already have an account? Balance Chemical Equation - Online Balancer. This method separates the reaction into two half-reactions — one for oxidation and one for reduction. Enter a chemical equation to balance:. Let's balance this equation using the algebraic method. Millions of real past notes, study guides, and exams matched directly to your classes.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait.
Gas laws. How to cite? Best for: Equations that are more complex and not easily balanced by inspection. What are the two products of the reaction? Contact us. No Try it. I have been a tutor online since , working at Got It! Write a chemical equation for the reaction. Enter either the number of moles or weight for one of the compounds to compute the rest. Balance the changes using electrons: Multiply the number of calcium atoms by 3 and the number of phosphorus atoms by 2.

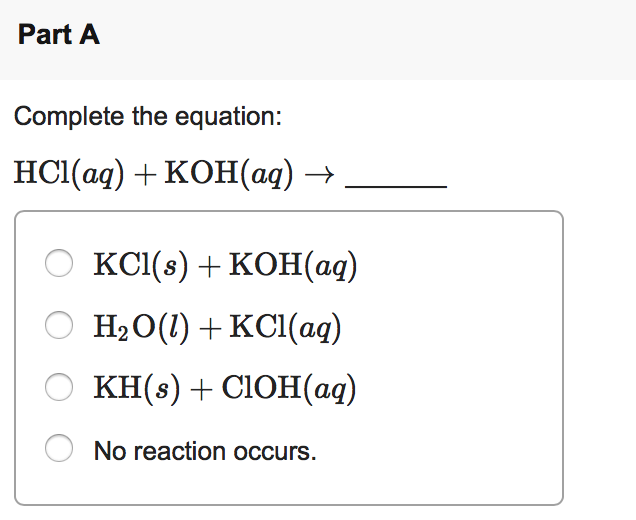
In it something is. I will know, many thanks for an explanation.
You are not right. I suggest it to discuss. Write to me in PM, we will communicate.