H2so3 compound name
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water.
Route of exposure:. Biological location:. Organ and components:. Biofluid and excreta:. Indirect biological role:. Biological role:.
H2so3 compound name
Molar mass of H 2 SO 3 Sulfurous acid is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
It can reduce metals to their elemental form.
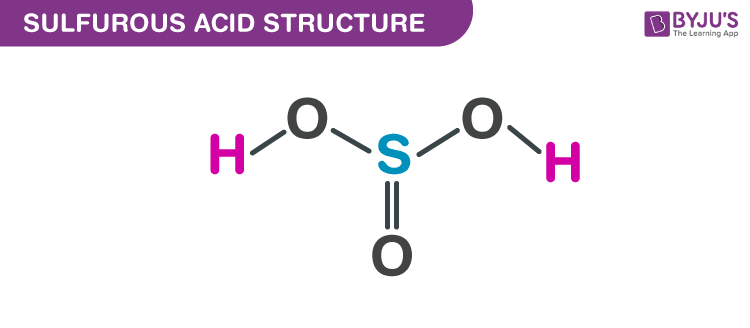
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses?
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term "acid test" arose from the California gold rush in the late s, when this combination was used to test for the presence of real gold. It has since come to mean "tested and approved" in a number of fields. An acid can be defined in several ways. This is a different type of compound than the others we have seen so far.
H2so3 compound name
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term "acid test" arose from the California gold rush in the late 's when this combination was used to test for the presence of real gold. It has since come to mean, "tested and approved" in a number of fields. An acid can be defined in several ways.
Germanyum faydaları
Property Value Reference Physical state Gas. Some are oxidizers and may ignite combustibles wood, paper, oil, clothing, etc. CAS Number. Toxicity of Sulphurous Acid Apart from sulphurous acid uses, there are some toxicity issues. The difference between oxidizing and non-oxidizing acids can be attributed to the electron-donating or electron-withdrawing nature of their respective functional groups. Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. GHS labelling :. Acidity p K a. What is name for acid H2SO3? On inhalation, sulphurous acid can irritate your throat and nose. Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts.
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide.
It can also cause inflammation of the lungs influenced by inhalation of toxic gases and vapours or metal fumes. What is Iodoform? As a bleaching agent, sulfurous acid is used for whitening wool, silk, feathers, sponge, straw, wood, and other natural products. What is the chemical name for H2SO2? Incompatible with strong bases. Shanghai Hanhong Scientific Co. And for H2SO4 it is hydrogen Sulphate. These are Method 1: By using sulphur dioxide. How to cite? Need Help? No Yes.

I agree with told all above. Let's discuss this question. Here or in PM.
Excellent idea
Curious question