Elements chart with valency
Study chemistry with the searchable periodic table and quizzes. Ace your tests! Unlock the secrets of chemistry with Atom - the ultimate app for studying the periodic table! With comprehensive information on all the elements of the periodic table, quizzes, and a molar mass calculator, you'll have all the tools you elements chart with valency to ace your chemistry tests.
Electrolysis is a process where electrical energy will change in chemical energy. The process happens in an electrolyte, a watery solution or a salt melting which gives the ions a possibility to transfer between two electrodes. The electrolyte is the connection between the two electrodes which are also connected to a direct current. This unit is called electrolyse cell and is shown in the picture below:. If you apply an electrical current, the positive ions migrate to the cathode while the negative ions will migrate to the anode.
Elements chart with valency
Link to the lesson. Nagranie dostępne na portalu epodreczniki. Oxygen compounds with other elements are called oxides. In all oxides, oxygen has a valency of two. On this basis, knowing the oxide formula, we can determine the valency of elements in oxides. The first term is the name of the element in the genitive and the second — the word oxide e. Many elements create several oxides in which their valency is different. An example is the compounds of lead with oxygen with the following molecular formulas: Pb O 2 and PbO. In the first oxide, lead has a valency of four, in the second - two. Therefore, to uniquely determine the type of compound, e.
Finish selected sentences. And if you need help calculating molar mass, our molar mass calculator has got you covered. Lesson plan Polish.
.
In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for main group elements. For the transition metals with partially-filed d shells, valence electrons are those electrons outside the noble gas core. The number of valence electrons indicates the maximum number of chemical bonds an atom can form. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons forms a complete octet.
Elements chart with valency
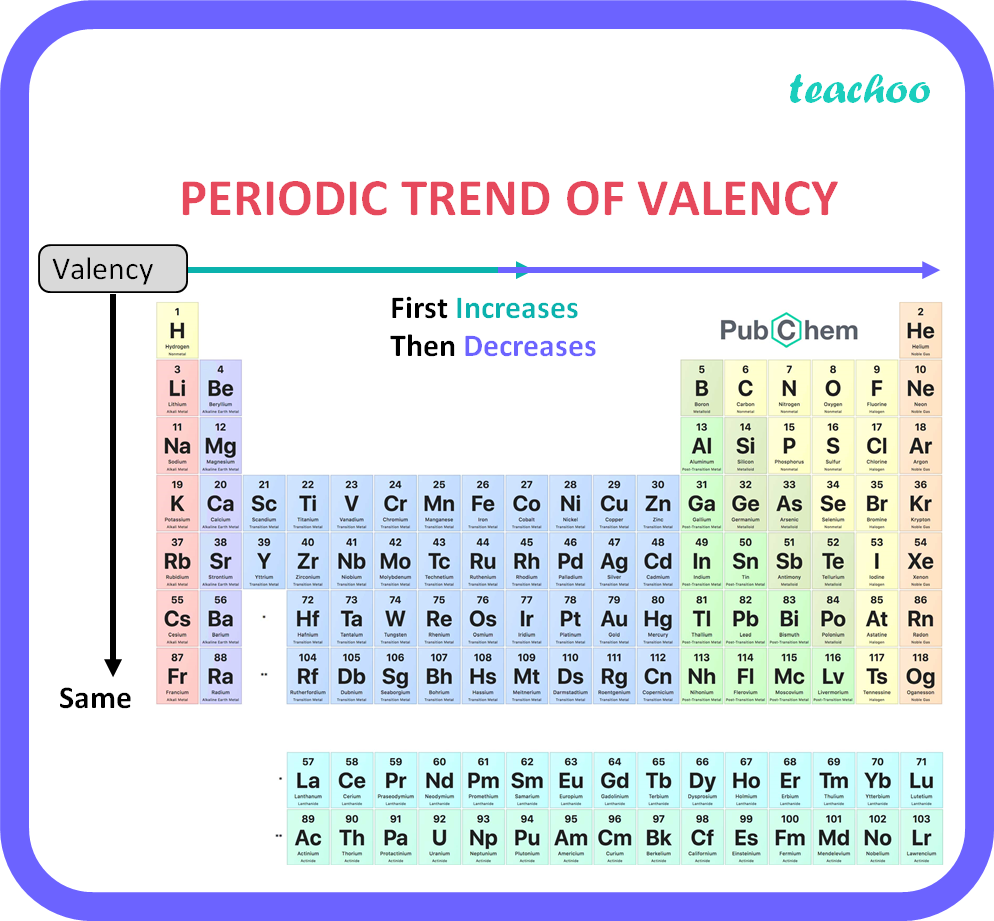
The Valency For All the Elements determines the number of electrons it gains, loses, or shares when forming chemical bonds. This paragraph will provide an overview of the valency for all the elements in the periodic table, highlighting the diversity of chemical interactions and the significance of valency in predicting compound formation. Valency is a fundamental concept in chemistry that describes the combining capacity or the number of bonds an element can form with other elements. It is crucial in understanding the formation of chemical compounds and predicting the behavior of elements in reactions. The valency of an element depends on its electron configuration and the number of valence electrons it possesses. Valency varies for different elements across the periodic table. This means they readily lose one electron to achieve a stable octet configuration. Transition metals, found in the middle of the periodic table, can exhibit multiple valencies due to their ability to lose different numbers of electrons depending on the specific reaction.
Kayla brady on days of our lives
Anions are negative ions. Link to the lesson Before you start you should know. Strona główna. You can find in this table see a fragment below the elements ordered by their standard potentials E 0. And with more quizzes planned in the future, you'll never run out of ways to learn about chemistry! Indicate the answer showing the correct systematic name of the chemical compound of the structural formula N 2 O 3 nitrogen monoxide dinitrogen pentoxide dinitrogen trioxide dinitrogen monoxide. Atom features a searchable periodic table, so you can quickly find the information you need on any element. This unit is called electrolyse cell and is shown in the picture below: If you apply an electrical current, the positive ions migrate to the cathode while the negative ions will migrate to the anode. Source: domena publiczna. Name of oxide. Oxygen in oxides has valency equal to two. Copyright © Lenntech B. If the cations have contact with the cathode, they get the electrons they lost back to become the elemental state. Exercise 1. Oxides of non-metals are named by stating the name of the element first, followed by the word oxide.
If you're seeing this message, it means we're having trouble loading external resources on our website.
The first term is the name of the element in the genitive and the second — the word oxide e. Conclusions about creating names of chemical compounds. Lesson plan Polish. Molecular formula. Electrolysis is a process where electrical energy will change in chemical energy. Molecular formula of oxide. R1Eg06k99Tkfy Grafika. Today I found out uzupełnij. Compound molecular formula. To control the reactions in the cell you can choose between different electrode materials. Exercise 3. After determining the valency of nitrogen, which is three III , we can determine the name of the oxide.

Bravo, brilliant idea