Electron withdrawing groups list
Homework problems?
Hey there! We receieved your request. Electron withdrawing groups through resonance effect:. Electron donating groups through resonance effect:. Please choose valid name. Please Enter valid email.
Electron withdrawing groups list
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next. What is Scribd? Academic Documents. Professional Documents. Culture Documents. Personal Growth Documents. Uploaded by jaanabhenchod. Document Information click to expand document information electron donating and electron withdrawing groups for quick study,.
Practice Accuracy.
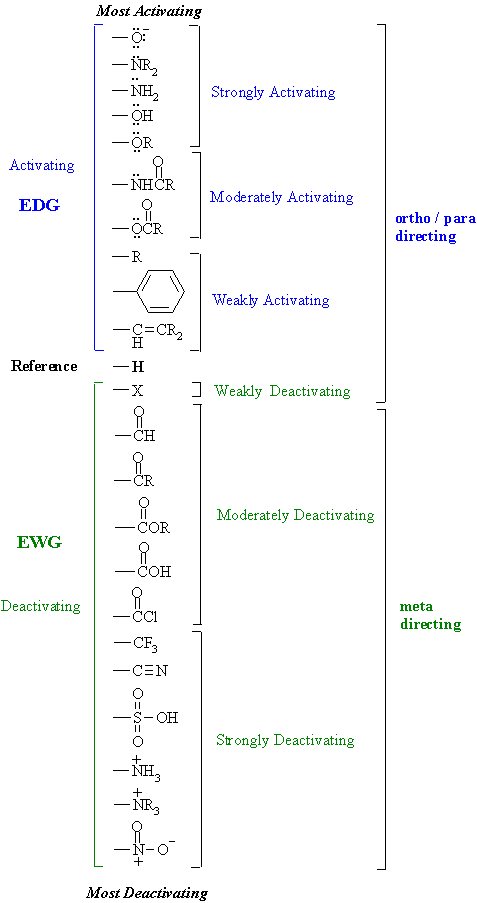
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction. An electron withdrawing group EWG will have the opposite effect on the nucleophilicity of the ring. EDGs and EWGs also determine the positions relative to themselves on the aromatic ring where substitution reactions are most likely to take place. Electron donating groups are typically divided into three levels of activating ability The "extreme" category can be seen as "strong". Electron withdrawing groups are assigned to similar groupings.
A substituent on a benzene ring can effect the placement of additional substituents on that ring during Electrophilic Aromatic Substitution. How do we know where an additonal substituent will most likely be placed? The answer to this is through inductive and resonance effects. Inductive effects are directly correlated with electronegativity. Substituents can either be meta directing or ortho-para directing.
Electron withdrawing groups list
The above reaction would more readily proceed if the electrophilicity of the carbonyl carbon were enhanced. This may be achieved through electron withdrawal via the R group. The ether -OMe , the methyl -Me , and the hydroxyl -OH , would all produce a electron-donating effect, and are thus incorrect answers. The nitro group -NO 2 , and the positively charged, tetra-substituted amino group consider the structure once this trimethyl amino group is connected to the aryl ring are both electron-withdrawing.
Double vanity bathroom ideas
Uploaded by jaanabhenchod. Hey there! Get quick access to the topic you're currently learning. Retrieved For example, aniline has resonance structures with negative charges around the ring system:. Last Viewed. Anesthesia Anesthesia. The inductive and resonance properties compete with each other but the resonance effect dominates for purposes of directing the sites of reactivity. From the course view you can easily see what topics have what and the progress you've made on them. Retrieved 2 April Organic Tute 8 Organic Tute 8. In the case of a fluorine substituent, for instance, the ortho partial rate factor is much smaller than the para , due to a stronger inductive withdrawal effect at the ortho position.
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction.
Retrieved 2 April See the image below: To find -M groups, look for double bonds to oxygen and nitrogen! To understand the underlying electronic effects that produce these properties. List of electronic donating group and electron withdrawing group List of electronic donating group and electron withdrawing group. You will get reply from our expert in sometime. Nitration and aromatic reactivity. By increasing electron density on adjacent carbon atoms, EDGs change the reactivity of a molecule: EDGs make nucleophiles stronger. Hey there! Although the full electronic structure of an arene can only be computed using quantum mechanics , the directing effects of different substituents can often be guessed through analysis of resonance diagrams. Click Here Know More. It is due to the higher reactivity of phenolate anion. Organic Tute 8 Organic Tute 8. Weakly deactivating groups direct electrophiles to attack the benzene molecule at the ortho- and para- positions, while strongly and moderately deactivating groups direct attacks to the meta- position. A carbon atom with a larger coefficient will be preferentially attacked, due to more favorable orbital overlap with the electrophile.

Excuse, that I interfere, there is an offer to go on other way.
Completely I share your opinion. It seems to me it is excellent idea. Completely with you I will agree.