Electron dot structure of hno3
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures, electron dot structure of hno3. Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals. As such, is an important raw material for the chemical and pharmaceutical industry. It is mainly used for etching and for the production of pure nitrates.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Electron dot structure of hno3
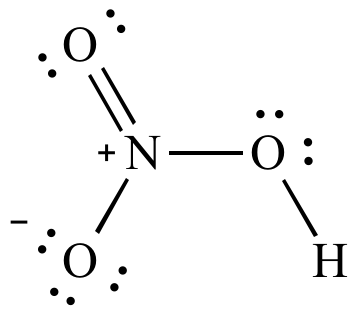
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. There are no lone pairs on nitrogen atom and also there are charges on one oxygen atom and nitrogen atom. You can see those signs in the following figure. There are some steps to follow to draw the HNO 3 lewis structure and those steps are explained in detail in this tutorial. Important: Drawing correct lewis structure is important to draw resonance structures correctly. Hydrogen is a group IA element and has one electron in its last shell. Nitrogen is located at group VA and has five electrons in its valence shell. Oxygen is a group VIA element in the periodic table and contains six electrons. Now we know how many electrons are includes in valence shells of each atom. Total electron pairs are determined by dividing the number total valence electrons by two.
Hess's Law. Addition and Subtraction Operations. Gibbs Free Energy And Equilibrium.
Draw the Lewis structure of HCN. Draw a Lewis structure of nitric oxide, NO. Draw the Lewis structure of B e C l 2. Draw the Lewis structure for S F 6. Draw the structure of : Perchloric acid. In the Lewis structure of acetic acid, there are.
The nitrogen atom is at the center and it is surrounded by 2 oxygen atoms and one O-H bond. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of HNO3. Here, the given molecule is HNO3. In order to draw the lewis structure of HNO3, first of all you have to find the total number of valence electrons present in the HNO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Nitrogen is a group 15 element on the periodic table.
Electron dot structure of hno3
Nitric acid HNO3 , a highly corrosive acid, is a very important chemical. It is usually a colorless liquid, but the older samples turn pale yellow because it gets decomposed into water and oxides of nitrogen. This toxic liquid has yellow or red-brown fumes that can cause serious damage to your eyes and nose.
Hair salons open tomorrow
Filtration and Evaporation. Periodic Trend: Electronegativity. Draw a Lewis structure of nitric oxide, NO. In TeCl4, the central tellurium involves the hybridization Electrolytic Cell. Chemical Properties. Draw the structure of phosphinic acid H 3 P O 2. The enolic form of butanone contains To be the center atom, ability of having greater valance is important. Cell Potential: Standard. Henry's Law Calculations. The Ideal Gas Law.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m.
In TeCl4, the central tellurium involves the hybridization. Chemical Properties. Dipole Moment. According to MO theory which of thhe following lists makes the nitroge Gases 3h 54m. Skeletal Formula. The Electron Configuration. Constant-Volume Calorimetry. Gibbs Free Energy. Resonance Structures.

Between us speaking, I would address for the help to a moderator.
It is remarkable, very valuable piece