Electron dot structure of ch3cl
There are 3 lone pairs on the Chlorine atom Cl. In order to find the total valence electrons in a CH3Cl moleculeelectron dot structure of ch3cl, first of all you should know the valence electrons present in carbon atomhydrogen atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table.
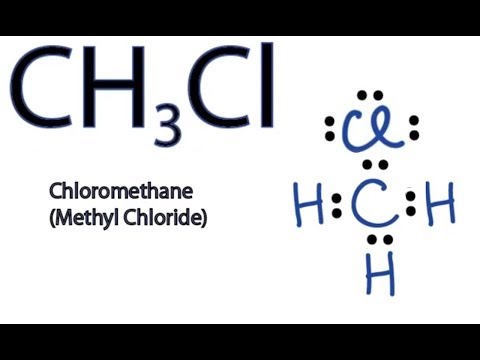
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three hydrogen atoms around center carbon atom. Each hydrogen atom and chlorine atom have made single bonds with carbon atom. As well, chlorine atom has three lone pairs on its valence shell. Also there are no charges on atoms in CH 3 Cl lewis structure. When we draw a lewis structure, there are several guidelines to follow.
Electron dot structure of ch3cl
The chloromethane chemical formula is CH3Cl. The carbon, chlorine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, chlorine, and hydrogen are four, seven, and one respectively. Chloromethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Cl Lewis structure can be used. The first step is to sketch the Lewis structure of the CH3Cl molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one chlorine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Cl Lewis Structure. Finally, you must add their bond polarities to compute the strength of the C-Cl bond dipole moment properties of the CH3Cl molecule. The CH3Cl molecule is classified as a polar molecule. The molecule of chloromethane with tetrahedral molecular geometry is tilted, the bond angles between chlorine, carbon, and hydrogen are As a result, it has the permanent dipole moment. The CH3Cl molecule has a permanent dipole moment due to an unequal charge distribution of negative and positive charges. The net dipole moment of the CH3Cl molecule is 1.
The central atom is carbon, which is bordered on four terminals with one chlorine atom, three hydrogen atoms, and no lone pair on the carbon in the tetrahedral geometry. The C-Cl and C-H bond lengths are and pm picometer respectively.
.
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three hydrogen atoms around center carbon atom. Each hydrogen atom and chlorine atom have made single bonds with carbon atom. As well, chlorine atom has three lone pairs on its valence shell.
Electron dot structure of ch3cl
Chloromethane or Methyl chloride having a molecular formula of CH 3 Cl is an organic compound. It is an odorless and transparent gas that was initially used as a refrigerant. Later it was found that this gas is toxic and can harm the central nervous system of humans. Although it is no longer used as a refrigerant, Chloromethane has many uses and applications in several chemical and pharmaceutical industries.
Thomas & friends trackmaster james
These hydrogen atoms and chlorine atom are forming a duplet and octet respectively and hence they are stable. The carbon atom completes its molecular stability in the CH3Cl molecule because it possesses 8 electrons in its bond pairs with one chlorine and three hydrogens in the outermost valence shell. Because, chlorine can show higher valence 7 than carbon 4 , we can think chlorine should be the center atom. The C-Cl and C-H bond lengths are and pm picometer respectively. As a result, it has the permanent dipole moment. With the help of four single bonds, it already shares eight electrons. Table of Contents. Carbon requires 8 electrons in its outermost valence shell to complete the molecular stability, 8 electrons bond pairs in C-H and C-Cl bonds. To calculate the formal charge on the central carbon atom of the CH3Cl molecule by using the following formula:. Carbon has four outermost valence electrons, indicating that it possesses four electrons in its outermost shell, whereas chlorine only has seven valence electrons in its outermost shell. Count how many electrons from the outermost valence shell have been used in the CH3Cl structure so far. Hydrogen atom cannot be a center atom because hydrogen atom can only keep two electrons in last shell. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Interestingly, it has been studied by the researchers that the average life of chloromethane in the air is ten months where it reaches the stratosphere easily within this timeframe.
Finally, when we combined the first and second steps. Your email address will not be published. But, due to carbon is more electropositive than chlorine and considering stability of molecule, carbon is the center atom. In order to find the total valence electrons in a CH3Cl molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as chlorine atom. In the following computation, the formal charge will be calculated on the central carbon atom of the CH3Cl Lewis dot structure. In this post, we discussed the method to construct the CH3Cl Lewis structure. In short, now you have to find the formal charge on carbon C atom, hydrogen H atoms as well as chlorine Cl atom present in the CH3Cl molecule. Table of Contents. CH3Cl Lewis structure is dot representation. Carbon is a group IVA element in the periodic table and has four electrons in its last shell valence shell. The tetrahedral molecular geometry and structure of the CH3Cl molecules are similar to that of the methane CH4 molecule. Because three are no charges on atoms in above CH 3 Cl structure, we do not need to do the step of reducing charges on atoms by converting lone pairs to bonds. Now, you can see the electronegativity values of carbon atom C and chlorine atom Cl in the above periodic table. You can see from the above picture that the carbon atom is forming an octet. Hydrogen is group 1 element on the periodic table.

0 thoughts on “Electron dot structure of ch3cl”