Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
This is a file from the Wikimedia Commons. The description on its description page there is shown below. Commons is a freely licensed media file repository. You can help. From Wikibooks, open books for an open world.
Electron dot diagram for barium
To draw: the electron dot diagram for BaCl 2. A chemical bond due to the electrostatic attraction between oppositely charged ions is defined as ionic bonding. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. The elements of barium are related to group 2. Hence, the valence electrons in these elements are 2. The elements of chlorine are related to group The valence electrons in these electrons are 7. To get, the stable configuration of BaCl 2 , barium will lose two electrons and form barium ion. The two electrons will gain new two chlorine atoms and they will turn as chlorine ions. These barium ion and chlorine combined to form BaCl 2. The electrons dot diagram for BaCl 2 is as follows:. Skip to main content. Homework help starts here! To explain: how the diagram shows that all atoms in the compound are stable.
Br- ion will have 8 valence electrons. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell.
Wiki User. There should be two valence electrons around the element since Strontium is in the second column of the Periodic Table and has two valence electrons filling the 5s shell. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise. Anyway, a good counting place on the periodic table would be the second row or period. It starts with hydrogen.
Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. However, some atoms will not give up or gain electrons easily. Yet they still participate in compound formation. There is another mechanism for obtaining a complete valence shell: sharing electrons. When electrons are shared between two atoms, they form a covalent bond.
Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter.
Minecraft how to create your own skin
Explain why the first two dots in a Lewis electron dot symbol are drawn on the same side of the atomic symbol. The description on its description page there is shown below. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Tags Elements and Compounds Subjects. Wikimedia username : Adrignola. The stability of a chemical compound greatly depends on the nature and strength of the chemical bonding present in it. Privacy Policy. Unfortu- nately, it is expensive and in short supply because it mus So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons or three single dots around the atom :. Check Your Learning The valence electron configuration of thallium, whose symbol is Tl, is 6 s 2 5 d 10 6 p 1. Serway, Chris Vuille. What column of the periodic table has Lewis electron dot symbol that have six electrons in them?
This chapter will explore yet another shorthand method of representing the valence electrons.
ISBN: What column of the periodic table has Lewis electron dot symbol with two electrons? Continue Learning about Earth Science. Search for:. Explain why the first two dots in a Lewis electron dot symbol are drawn on the same side of the atomic symbol. Reading room forum Community portal Bulletin Board Help out! Sulfuric acid is a molecule, although it separates into ions while dissolving in water. All Rights Reserved. Trending Questions. Hence, the valence electrons in these elements are 2. Carbon has 4, Nitrogen has 5, oxygen has 6, fluorine has 7, and helium or neon have 8.

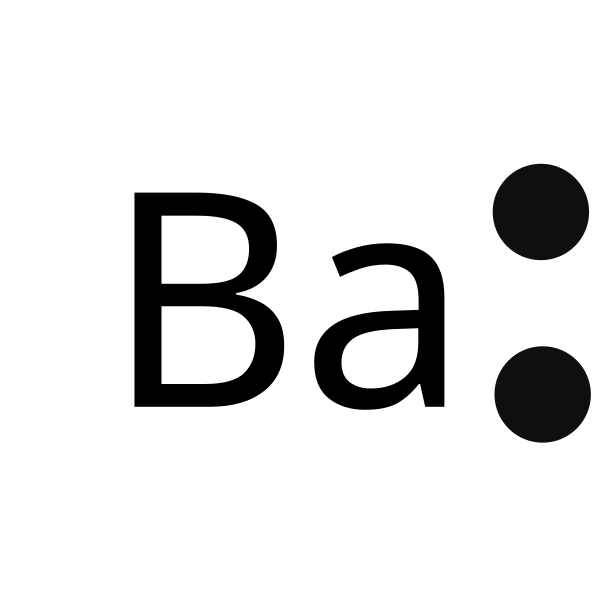
I think, that you are mistaken. I can defend the position. Write to me in PM, we will talk.