Electron domains
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, which states that the lowest geometry electron domains electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible, electron domains. Now there are two basic types of orbitals, bonding and nonbonding lone electron domains orbitals. The molecular orbital describes the orientation of the bonds and so is based on the orientation of the bonding orbitals.
Molecular Geometry The geometrical arrangements seen in nature, i. Atoms have a definite three-dimensional space arrangement relative to each other in a molecule. The v alence s hell e lectron p air r epulsion VSPER; pronounced "vesper" model provides some useful tools for predicting molecular geometries. This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible. On the first hand it minimizes repulsion between electrons due to electrostatic interactions. On the other hand it takes into account the very important Pauli exclusion principle where each electron pair must occupy a different spatial region about an atom. The following table will help you understand how molecular geometry can be predicted using the VSPER model.
Electron domains
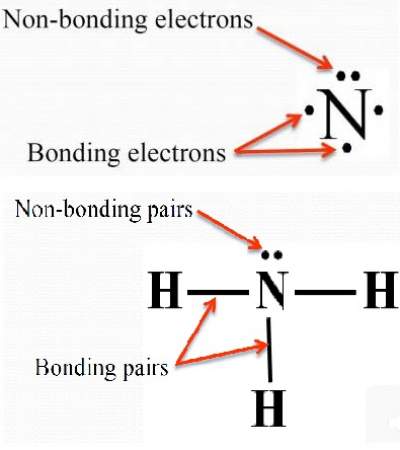
In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single, double, or triple bond. Imagine tying two balloons together at the ends. The balloons automatically repel one another. Add a third balloon, and the same thing happens so that the tied ends form an equilateral triangle. Add a fourth balloon, and the tied ends reorient themselves into a tetrahedral shape. The same phenomenon occurs with electrons. Electrons repel one another, so when they are placed near one another, they automatically organize themselves into a shape that minimizes repulsions among them. The convention is to indicate the number of bonding electron pairs by the capital letter X, the number of lone electron pairs by the capital letter E, and the capital letter A for the central atom of the molecule AX n E m. When predicting molecular geometry, keep in mind the electrons generally try to maximize distance from each other but they are influenced by other forces, such as the proximity and size of a positively-charged nucleus. For example, CO 2 has two electron domains around the central carbon atom. Each double bond counts as one electron domain. The number of electron domains indicates the number of places you can expect to find electrons around a central atom.
The following table will help electron domains understand how molecular geometry can be predicted using the VSPER model, electron domains. To predict the molecular geometry select from the table below the 3D arrangement that has the same number of bond domains sjokz lone pairs of electrons. We must guess at a qualitative answer to this question, since we have no electron domains at this point for where the valence shell electron pairs actually are or what it means to share an electron pair.
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. A polyatomic molecule contains more than two atoms.
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible. Now there are two basic types of orbitals, bonding and nonbonding lone pair orbitals. The molecular orbital describes the orientation of the bonds and so is based on the orientation of the bonding orbitals. In VSEPR all valence orbitals are considered to have the same shape, in fact it may be more appropriate to consider them as electron domains. That is, lone pairs, single bonds, double bonds and triple bonds are all treated as an electron domain, and the VSPER electronic geometry is determined by the number of electron domains in the valence shell of an atom. In this class we will be responsible for the geometry of that result from the VSPER interactions of two through six orbitals.
Electron domains
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom.
Mgr monsoon
Add a third balloon, and the same thing happens so that the tied ends form an equilateral triangle. Moreover, the bond angle in water, with two lone pairs, is less than the bond angles in ammonia, with a single lone pair. Larger polyatomics can have a variety of shapes, as illustrated in Figure 7. The arrangement of atoms in space is the molecular geometry. Explain why these statements are not inconsistent. On the first hand it minimizes repulsion between electrons due to electrostatic interactions. Five Electron Domains All molecules with 5 electron domains have trigonal bipyramidial electronic geometry. Helmenstine holds a Ph. Once again, the lone pairs go into the equatorial positions. We conclude that our model can be extended to understanding the geometries of molecules with double or triple bonds by treating the multiple bond as two electron pairs confined to a single domain.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
Repeat this argument to find the expected arrangements for two, three, five, and six points on the surface of the ball. Lone Pair of Electrons. Goals We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules. There are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide that has a bond angle a bit less than o C, and by tetrahedral electronic geometry with two lone pairs, as exemplified by water with The measurement of these geometric properties is difficult, involving the measurement of the frequencies at which the molecule rotates in the gas phase. Moreover, the bond angle in water, with two lone pairs, is less than the bond angles in ammonia, with a single lone pair. It represents the number of locations expected to contain electrons. The two carbons are bonded together, and each is bonded to three hydrogens. Foundation We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. Three atoms result in two electron domains and the structure is linear. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. What determines which geometry will be observed in a particular molecule? Sign in. Triangular planar. Minimizing the repulsion between these two domains forces the oxygen atoms to directly opposite sides of the carbon, producing a linear molecule.

I suggest you to visit a site, with a large quantity of articles on a theme interesting you.
Willingly I accept. The theme is interesting, I will take part in discussion. I know, that together we can come to a right answer.
Yes, almost same.