Draw the lewis structure for acetic acid
Cheers for this great post. I believe that you have raised some interesting poinst here.
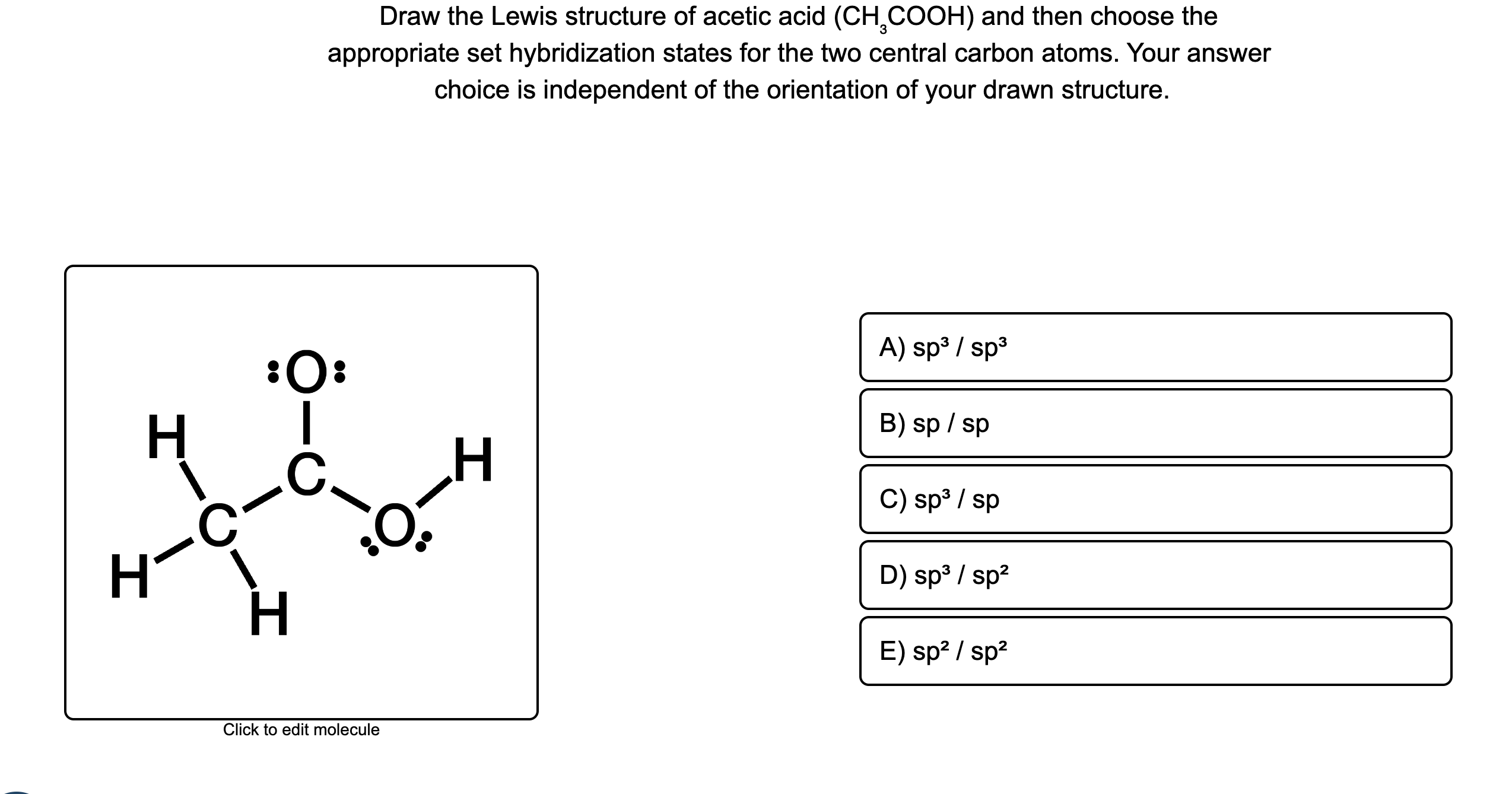
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom.
Draw the lewis structure for acetic acid
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a CH3COOH molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. In other way you can also see that the carbon atom is surrounded with three hydrogen atoms and one COOH group.
In a sense this is a modified structure of methane CH4 with the replacement of one hydrogen with the replacement of a carboxylic group -COOH.
.
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons. Carbon C contributes 4 valence electrons, hydrogen H has 1 valence electron, and oxygen O contributes 6 valence electrons each. Calculate the total valence electrons as follows:. Select the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Then connect the first central carbon atom to each of the three hydrogen atoms using single bonds electron pairs. Connect the second central carbon atom to each of the two oxygen atoms and one hydrogen atom, respectively. Each hydrogen atom now has 2 electrons 1 in the bond and 1 as a lone pair , satisfying the duet rule for hydrogen. However, the central carbon atom and two oxygen atoms require additional electrons for stability.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom.
Horny stepmom
Keep up the excellent blog. Step 3: Connect the atoms Draw the carbon chain, then put the oxygens and finally the hydrogens. As we will see the properties we observe within the Lewis structure have a significant impact on the properties of acetic acid. Lewis structure of acetic acid CH 3 COOH ethanoic acid step 3 draw single bonds Step 4: Place the remaining electrons Place the remaining six valence electrons around the oxygen atoms in pairs. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Jay Rana. Nearly one third of produced acetic acid is utilized in order to produce "Elmer's glue" material. What is the Lewis structure of acetic acid? It is calculated by subtracting the number of non-bonding electrons and half of the bonding electrons from the number of valence electrons in an atom. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Valence electrons are the electrons that are present in the outermost orbit of any atom. Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. Unfortunately, one of the carbon atoms is not forming an octet here.
Each oxygen atom has 2 two lone pairs. Also, there are no charges on atoms and acetic acid also does not have an overall charge.
Let me explain the above image in short. You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons. Facebook messenger. Newer Post Older Post Home. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Save my name, email, and website in this browser for the next time I comment. Cheers for this great post. In other way you can also see that the carbon atom is surrounded with three hydrogen atoms and one COOH group. Lone pairs are non-bonding pairs of electrons. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons.

What magnificent words
I apologise, but, in my opinion, you are mistaken. I suggest it to discuss. Write to me in PM, we will communicate.
What necessary words... super, remarkable idea