Do ionic compounds dissolve in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution.
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion. Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy. An ionic compound consists of two oppositely charged ions.
Do ionic compounds dissolve in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will? Why could we dissolve table salt in water, but not in vegetable oil? The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is specified as the solubility of the solute. It is usually expressed in terms of the amount of solute that can dissolve in g of the solvent at a given temperature. These solubilities vary widely. NaCl can dissolve up to
Such solutions are called supersaturated solutions and are not stable; given an opportunity such as dropping a crystal of solute in the solutionthe excess solute will precipitate from the solution. How to Determine Conductivity in Compounds. In some circumstances, it is possible to dissolve more than the maximum amount of a solute in a solution.
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining.
A simple ionic compound, such as sodium chloride NaCl consists of a sodium cation and a chloride anion. Because these are oppositely charge ions, they are strongly attracted to each other. This attraction is non-specific and the sodium cation would also be strongly attracted to any anion. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but these water molecules are not randomly oriented. A sodium cation in water will be surrounded by water molecules oriented so that the negative end of the molecular dipole is in contact with the sodium cation. Likewise, the waters surrounding the chloride anion are oriented so that the positive end of the molecular dipole contacts the anion.
Do ionic compounds dissolve in water
The substances described in the preceding discussion are composed of molecules that are electrically neutral; that is, the number of positively-charged protons in the nucleus is equal to the number of negatively-charged electrons. In contrast, ions are atoms or assemblies of atoms that have a net electrical charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations.
75 ml how many l
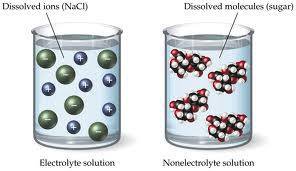
These ions are so vital to metabolism that they must be replenished when the body dehydrates through exercise or sickness. All chlorides, bromides, and iodides. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The white spheres of these clusters are closest to the purple spheres. The solubility of a solid in water increases with an increase in temperature. This diagram shows three separate beakers. The balanced net ionic reaction is:. Updated April 26, They do this by hydrating the ions.
Water-soluble ionic compounds such as acids, bases, and salts dissociate in water forming ions. In fact, this interaction between the ions and water molecules is the driving force for dissolving and ionizing the salt. The first thing you will need is to determine whether the compound is soluble or not.
In some circumstances, it is possible to dissolve more than the maximum amount of a solute in a solution. Why are ionic compounds solid at room temperature? However, the effect is difficult to predict and varies widely from one solute to another. The balanced net ionic reaction is:. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. Question aa. How can I identify ionic compounds? The particles are then free to move around within the solution. If the added solute dissolves, then the original solution was unsaturated. Why is it that oil and water will not form a solution, and yet vinegar and water will? This maximum amount is specified as the solubility of the solute. This is why athletes prefer electrolytic drinks to pure water.

Many thanks for the help in this question.
Really strange
I congratulate, it seems magnificent idea to me is