Diphosphate
Adenosine diphosphate ADPalso known as adenosine pyrophosphate APPdiphosphate, is an important organic compound in metabolism and is essential to the flow of diphosphate in living cells.
A number of pyrophosphate salts exist, such as disodium pyrophosphate Na 2 H 2 P 2 O 7 and tetrasodium pyrophosphate Na 4 P 2 O 7 , among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate , as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond. Pyrophosphoric acid is a tetraprotic acid, with four distinct p K a 's: [1].
Diphosphate
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'diphosphate. Send us feedback about these examples. Accessed 9 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! See Definitions and Examples ». Log In. Examples of diphosphate in a Sentence. Recent Examples on the Web This is only possible when there is enough of a binding molecule called geranyl diphosphate GPP. Word History. First Known Use. Time Traveler. See more words from the same year. Phrases Containing diphosphate. Dictionary Entries Near diphosphate. Cite this Entry.
Lehninger principles of biochemistry. Download as PDF Printable version. Phosphorus compounds.
.
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'diphosphate. Send us feedback about these examples. Accessed 9 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! See Definitions and Examples ». Log In.
Diphosphate
Phosphate is everywhere in biochemistry. As we were reminded in the introduction to this chapter, our DNA is linked by phosphate:. The function of many proteins is regulated - switched on and off - by enzymes which attach or remove a phosphate group from the side chains of serine, threonine, or tyrosine residues. Countless diseases are caused by defects in phosphate transferring enzymes. As just one example, achondroplasia, a common cause of dwarfism, is caused by a defect in an enzyme whose function is to transfer a phosphate to a tyrosine residue in a growth-related signaling protein. Finally, phosphates are excellent leaving groups in biological organic reactions, as we will see many times throughout the remainder of this book. Clearly, an understanding of phosphate chemistry is central to the study of biological organic chemistry.
Mercedes eqs wiki
ADP is stored in dense bodies inside blood platelets and is released upon platelet activation. Send us feedback about these examples. Retrieved See Definitions and Examples ». Gmelin Reference. The significance of ATP is in its ability to store potential energy within the phosphate bonds. Homophones, Homographs, and Homonyms. Agonists: 8-Aminoadenine Adenine. Pick the best ones! Main article: ATP synthase. The alkali metal salts are water-soluble. Glycolysis is performed by all living organisms and consists of 10 steps.
Is disodium phosphate dangerous? Disodium phosphate is a food additive.
Main article: ATP synthase. Gmelin Reference. Cite this Entry. Class of chemical compounds. Pyrophosphate Phosphonatophosphate. N verify what is Y N? The biosynthesis of ATP is achieved throughout processes such as substrate-level phosphorylation , oxidative phosphorylation , and photophosphorylation , all of which facilitate the addition of a phosphate group to ADP. The plasma concentration of inorganic pyrophosphate has a reference range of 0. PP i occurs in synovial fluid , blood plasma , and urine at levels sufficient to block calcification and may be a natural inhibitor of hydroxyapatite formation in extracellular fluid ECF. Phosphorus , P. Retrieved Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. The net reaction for the overall process of glycolysis is: [6].

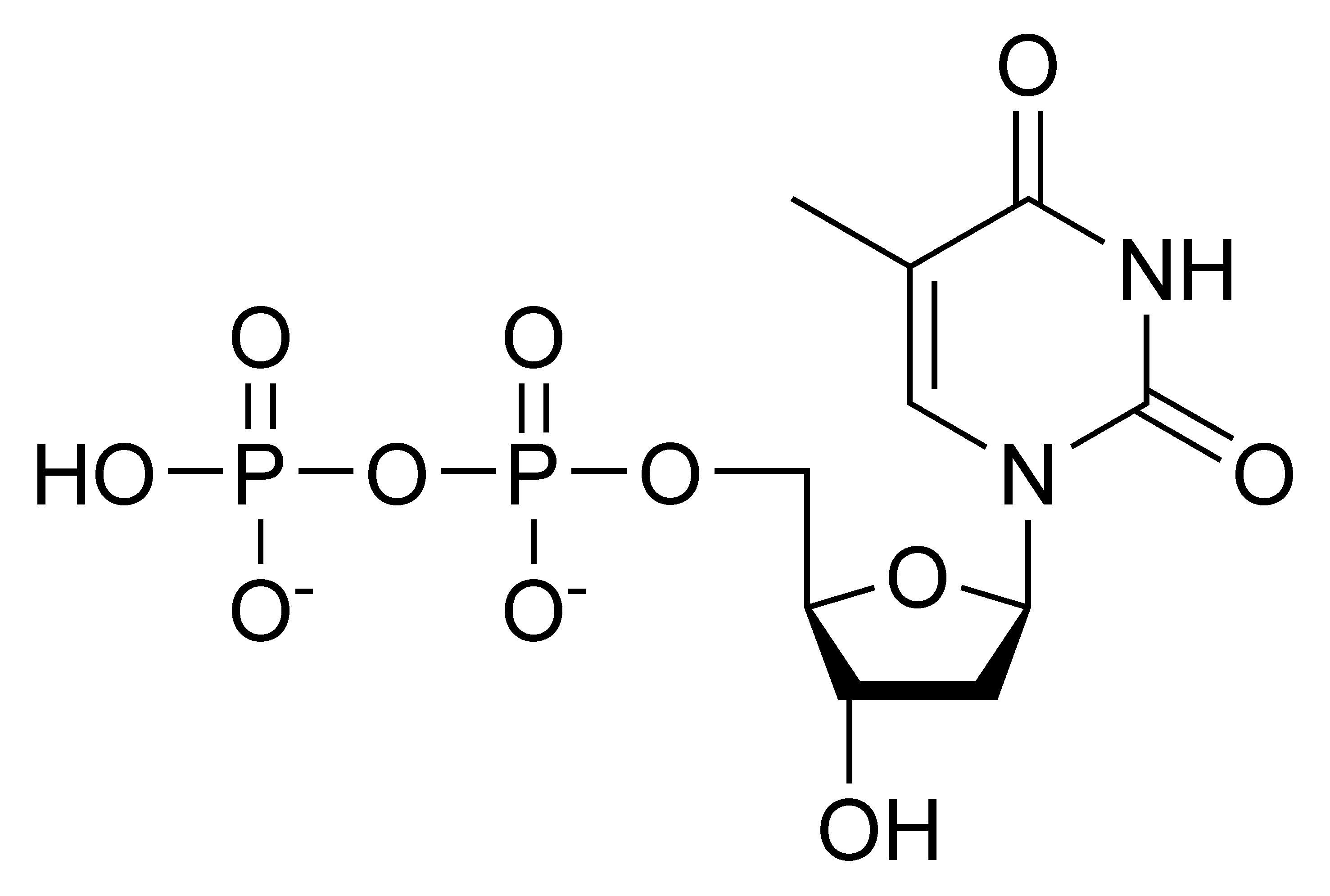
Willingly I accept. In my opinion it is actual, I will take part in discussion.
From shoulders down with! Good riddance! The better!