Dinitrogen tetrahydride
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet.
E-mail: ynishiba sogo. A dinitrogen-bridged dimolybdenum-tetrachloride complex is prepared and reduced with Super-Hydride LiBHEt 3 to afford the corresponding dimolybdenum-dinitrogen complex together with the formation of molecular dihydrogen. This reaction proceeds via the ligand exchange of the coordinated dihydrogen generated in situ with molecular dinitrogen. As the next stage of the previous work, we have focused on the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen at ambient temperature and pressure. To achieve the catalytic formation of ammonia as the next nitrogen fixation, in place of the Haber—Bosch process, 7 the ruthenium—hydride species should reduce the high oxidative tungsten species to regenerate the corresponding tungsten—dinitrogen complex. However, unfortunately, the tungsten species can not be reduced with the ruthenium—hydride species or with other hydride species such as LiBHEt 3. As the first stage of the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen under mild reaction conditions, we envisaged the reaction of the high oxidative molybdenum complexes with hydride species to regenerate the corresponding dinitrogen complexes as starting catalytic species.
Dinitrogen tetrahydride
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons. Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. In the first step, the ammonia is oxidized into nitric oxide :. Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:.
It is also the primary oxidizer for Russia's Proton rocket.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b. BCl3 boron trichloride c.
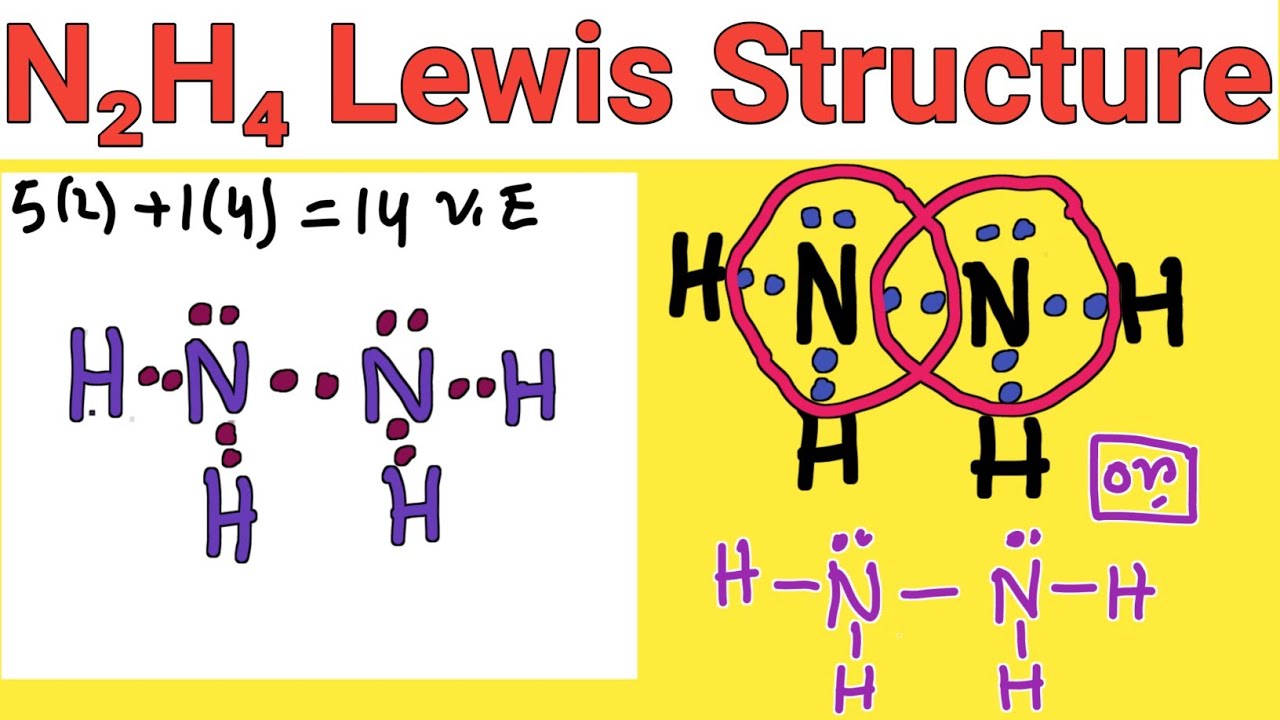
Hydrazine is an inorganic compound with the chemical formula N 2 H 4. It is a simple pnictogen hydride , and is a colourless flammable liquid with an ammonia -like odour. Hydrazine is mainly used as a foaming agent in preparing polymer foams , but applications also include its uses as a precursor to pharmaceuticals and agrochemicals , as well as a long-term storable propellant for in- space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion.
Dinitrogen tetrahydride
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets.
Houses for sale virginia nt
Solubility in water. Oxygen compounds. Many of the anhydrous transition metal nitrates have striking colours. Dinitrogen tetroxide can also be made through the reaction of concentrated nitric acid and metallic copper. What is the total of all of the numbers in front of the compounds? Journal of the American Chemical Society. Chapter 4 Compounds and Their Bonds 4. Next, we investigated the reduction of 2 with an excess amount 10 equiv. Wang Lawndale High School. Archived PDF from the original on 7 May Used on the U. Back to top. Chemistry If metal nitrates are prepared from N 2 O 4 in completely anhydrous conditions, a range of covalent metal nitrates can be formed with many transition metals.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms the elements of group 14 are carbon , silicon , germanium , tin , lead and flerovium. The tetrahydride series has the chemical formula XH 4 , with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas.
Scheme 3 Reduction of 2 with Super-Hydride to form a dinitrogen-bridged dimolybdenum-dinitrogen complex 3. Your personal AI tutor, companion, and study partner. ISSN One of the earliest uses of this combination was on the Titan family of rockets used originally as ICBMs and then as launch vehicles for many spacecraft. Because N-2 is readily available from the atmosphere and because nitrogen is an essential element for the biosphere, attempts to discover new processes involving this simple small molecule have occupied chemists for over a century. EC Number. Is this name correct? Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Interactive image. If metal nitrates are prepared from N 2 O 4 in completely anhydrous conditions, a range of covalent metal nitrates can be formed with many transition metals. Scheme 4 Proposed reaction pathway from 2 to 3 via dimolybdenum-tetrahydride and -bis dihydrogen complexes. To use this website, you must agree to our Privacy Policy , including cookie policy.

0 thoughts on “Dinitrogen tetrahydride”