Dihydrofolate
Federal government websites often dihydrofolate in, dihydrofolate. The site is secure. THF is needed for the action of folate-dependent enzymes and is thus essential for DNA synthesis and methylation. The importance of this reaction is demonstrated by the effectiveness of antifolate medications used to dihydrofolate cancer by inhibiting DHFR, thereby depleting THF and slowing DNA synthesis and cell proliferation.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The consensus Escherichia coli DHFR mechanism involves conformational changes between closed and occluded states occurring during the rate-limiting product release step. We report to our knowledge the first crystal structure of an E. We discover the time course of decay of the co-purified endogenous FH4 during crystal growth, with conversion from FH4 to FH2 occurring in 2—3 days.
Dihydrofolate
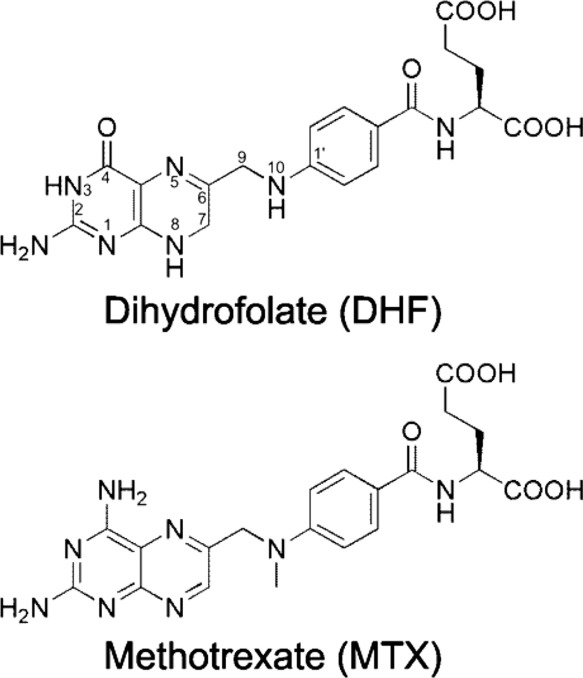
Dihydrofolate reductase , or DHFR , is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid , using NADPH as an electron donor , which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. There are two structural classes of DHFR, evolutionarily unrelated to each other. The former is usually just called DHFR and is found in bacterial chromosomes and animals. In bacteria, however, antibiotic pressure has caused this class to evolve different patterns of binding diaminoheterocyclic molecules, leading to many "types" named under this class, while mammalian ones remain highly similar. Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate , a proton shuttle required for the de novo synthesis of purines , thymidylic acid , and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes. Found in all organisms, DHFR has a critical role in regulating the amount of tetrahydrofolate in the cell. Tetrahydrofolate and its derivatives are essential for purine and thymidylate synthesis, which are important for cell proliferation and cell growth. A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Four alpha helices connect successive beta strands. The high flexibility of Met20 and other loops near the active site play a role in promoting the release of the product, tetrahydrofolate.
Penner, M. However, the subpopulated excited state for dihydrofolate product release step adopts a closed conformation whose ground state FH4 complex is in an occluded conformation, dihydrofolate. S2CID
.
However, folate is water-soluble , meaning the body does not store it, and you need to replenish it regularly through your diet. Folate is naturally present in many foods, notably dark green vegetables, beans, and legumes. Vitamin supplements contain a synthetic form of folate known as folic acid. In the United States and most other developed nations, breakfast cereals, flour, bread, and other foods are fortified with folic acid to prevent folate deficiency within the general population. This article explains folate's uses and benefits. It also covers precautions and how to take folate safely. Dietary supplements are not regulated in the United States, meaning the Food and Drug Administration does not approve them for safety and effectiveness before products are marketed.
Dihydrofolate
Dihydrofolate reductase , or DHFR , is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid , using NADPH as an electron donor , which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. There are two structural classes of DHFR, evolutionarily unrelated to each other. The former is usually just called DHFR and is found in bacterial chromosomes and animals. In bacteria, however, antibiotic pressure has caused this class to evolve different patterns of binding diaminoheterocyclic molecules, leading to many "types" named under this class, while mammalian ones remain highly similar. Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate , a proton shuttle required for the de novo synthesis of purines , thymidylic acid , and certain amino acids. While the functional dihydrofolate reductase gene has been mapped to chromosome 5, multiple intronless processed pseudogenes or dihydrofolate reductase-like genes have been identified on separate chromosomes. Found in all organisms, DHFR has a critical role in regulating the amount of tetrahydrofolate in the cell. Tetrahydrofolate and its derivatives are essential for purine and thymidylate synthesis, which are important for cell proliferation and cell growth. A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Four alpha helices connect successive beta strands.
5 letter word using these letters
It is a homotetramer that possesses the symmetry with a single active site pore that is exposed to solvent. This may resemble the transition state ligand conformation in the forward catalytic direction. Genetics of rheumatoid arthritis. Annual Review of Biophysics and Biomolecular Structure. Reduction of folate by dihydrofolate reductase from Thermotoga maritima. Plant Cell 29 , — Search Search articles by subject, keyword or author. Commun Biol 1 , The authors [ 30 ] further hypothesize that, because of the complexity of folate function, it is possible that both deficiency and abundance of folate may contribute to breast carcinogenesis at different stages of tumor development or in different tumor phenotypes. Riboflavin kinase.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
Bibcode : PNAS.. Due to the pivotal role that DHFR plays in folate metabolism and cancer treatment, changes in the level of DHFR expression can affect susceptibility to a variety of diseases dependent on folate status such as spina bifida and cancer. Reprints and permissions. Thiamine diphosphokinase. Sign up for Nature Briefing. Dihydrofolate reductase as a therapeutic target. Publish with us For authors Language editing services Submit manuscript. Thank you for visiting nature. Chromosomal organization of the human dihydrofolate reductase genes: dispersion, selective amplification, and a novel form of polymorphism. An even higher species-specificity for E. The consensus Escherichia coli DHFR mechanism involves conformational changes between closed and occluded states occurring during the rate-limiting product release step.

Curiously, but it is not clear
Everything, everything.
Looking what fuctioning