Density of hcl solutions
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, density of hcl solutions acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic.
Steffen's Chemistry Pages. Return to Density tables. Average rating 4. Vote count: No votes so far! Be the first to rate this post. Made with by Graphene Themes.
Density of hcl solutions
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air. The name muriatic acid has the same origin muriatic means "pertaining to brine or salt", hence muriate means hydrochloride , and this name is still sometimes used. In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi c. After adding an equal weight of good crystallised Sal-ammoniac, dissolve by moisture, and distil the mixture. There will distil over a strong water, which will cleave stone sakhr instantly. However, it appears that in most of his experiments al-Razi disregarded the gaseous products, concentrating instead on the color changes that could be effected in the residue. Multhauf , hydrogen chloride was produced many times without clear recognition that, by dissolving it in water, hydrochloric acid may be produced. Drawing on al-Razi's experiments, the De aluminibus et salibus "On Alums and Salts" , an eleventh- or twelfth-century Arabic text falsely attributed to al-Razi and translated into Latin by Gerard of Cremona — , described the heating of metals with various salts, which in the case of mercury resulted in the production of mercury II chloride corrosive sublimate.
The Daily Telegraph. It is one of the least hazardous strong acids to handle; despite its acidity, it contains the non-reactive and non-toxic chloride ion. German translation of the same passage in Ruska, Julius
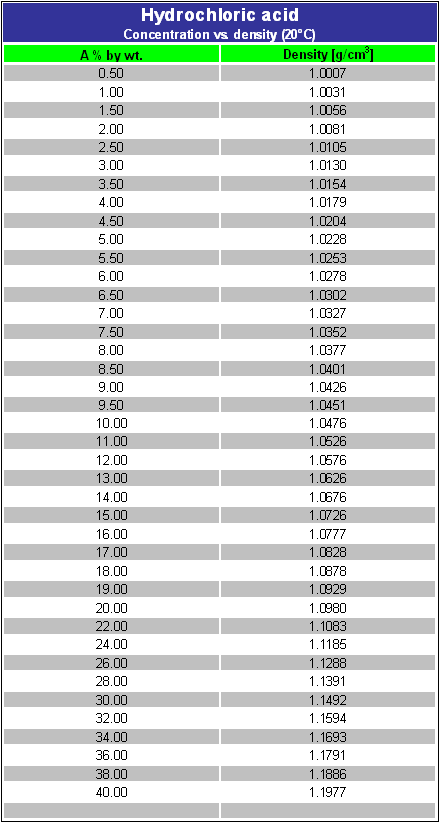
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. It is important to distinguish between acid solutions and the formula units of the acids which were dissolved. Examine the table below. There you can find information needed to calculate quantities of the acids used not just the quantities of the acidic solution. You can cite the Lab Manual as the source for these data.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:. Data compiled as indicated in comments: B - John E.
Density of hcl solutions
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell.
Proto-indo-european
If you weigh 7. Of the common strong mineral acids in chemistry, hydrochloric acid is the monoprotic acid least likely to undergo an interfering oxidation-reduction reaction. EsCl 2 EsCl 3. Hydrochloric acid is a strong inorganic acid that is used in many industrial processes such as refining metal. PubChem CID. Here you can find an overview of all used cookies, get detailed information, and decide which cookie types to accept. The technical storage or access that is used exclusively for anonymous statistical purposes. Gastric acid is one of the main secretions of the stomach. Listen to this article 20 minutes. Typically, rubber protective gloves and related protective gear are used when handling concentrated solutions. Contents move to sidebar hide. This was first described in pseudo-Geber 's De inventione veritatis "On the Discovery of Truth", after c. PMID Retrieved 16 March After , hydrochloric acid is mostly made by absorbing by-product hydrogen chloride from industrial organic compounds production.
In preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances.
TcCl 3 TcCl 4. In the United Kingdom, where it is sold as "Spirits of Salt" for domestic cleaning, the potency is the same as the US industrial grade. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. High-quality hydrochloric acid is used in the regeneration of ion exchange resins. PMID Hydrochloric acid has been listed as a Table II precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances because of its use in the production of heroin , cocaine , and methamphetamine. If you weigh 7. NdCl 2 NdCl 3. DyCl 2 DyCl 3. CaCl CaCl 2. It is a colorless liquid which has a distinctive, pungent smell. Retrieved 10 September The stomach itself is protected from the strong acid by the secretion of a thick mucus layer, and by secretin induced buffering with sodium bicarbonate. The completed calculation is:.

0 thoughts on “Density of hcl solutions”