Density of glycerin kg m3
List of these foods starting with the highest contents of Fiber, total dietary and the lowest contents of Fiber, total dietary. Calculate how much of this gravel is required to attain a specific depth in a cylindricalquarter cylindrical or in a rectangular shaped aquarium or pond [ weight to volume volume to weight price ]. Chloroperoxyl, gas [ClO 2 ] weighs 9. Volume to weightweight to volume and cost conversions for Refrigerant RD, liquid RD with density of glycerin kg m3 in the range of
It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups , glycerol is miscible with water and is hygroscopic in nature. Although achiral , glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a sn - prefix before the stem name of the molecule. Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides , esters of glycerol with long-chain carboxylic acids.
Density of glycerin kg m3
EC Safety Phrase. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. No special precautions indicated. Wash thoroughly after handling. Wash hands before eating. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash clothing before reuse. Skin: Wear appropriate protective gloves to prevent skin exposure.
It is also used as filler in commercially prepared low-fat foods e.
Submitted by Kevin N. Solved by verified expert. We will assign your question to a Numerade educator to answer. In a laboratory experiment, some glycerin is forced through a horizontal tube that is The high-pressure end of the tube is held at a gauge pressure of Pa, while the other end is open to the atmosphere. What is the flow rate of the glycerin through the tube?
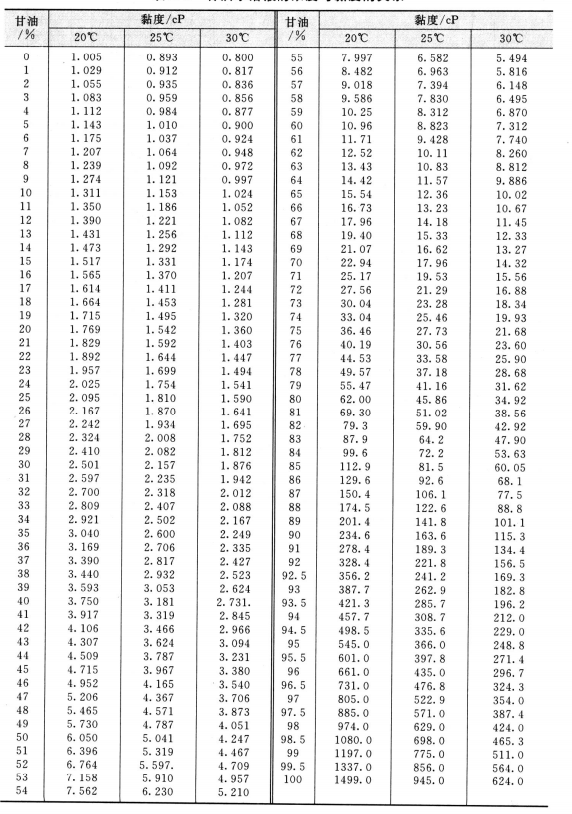
Can you tell me the exac t density of Glycerine? We will also teach you how to find it using the density formula. Glycerin is a common ingredient in pharmaceutical drugs, including heart medication, suppositories, cough remedies, and anaesthetics. Also, it is a type of carbohydrate called sugar alcohol or polyol. Glycerin is used in a variety of food and drinks products and also in skin-care, and cosmetic products. The density of Glycerin is 1. Glycerin is another name for glycerol, the three-carbon backbone of a triglyceride.
Density of glycerin kg m3
Note that even if pounds per cubic foot is often used as a measure of density in the U. Slugs are the correct measure of mass. You can divide pounds per cubic foot by
80s male dress up ideas
In other projects. Chemical formula. Video Answer Solved by verified expert. When the body uses stored fat as a source of energy, glycerol and fatty acids are released into the bloodstream. Retrieved 2 May Chloroperoxyl, gas [ClO 2 ] weighs 9. Q: Water with a density of In some organisms, the glycerol component can enter the glycolysis pathway directly and, thus, provide energy for cellular metabolism or, potentially, be converted to glucose through gluconeogenesis. So we developed a line of study tools to help students learn their way. Glycerol is a stable preserving agent for botanical extracts that, when utilized in proper concentrations in an extraction solvent base, does not allow inverting or mitigates reduction-oxidation of a finished extract's constituents, even over several years. For human consumption, glycerol is classified by the FDA among the sugar alcohols as a caloric macronutrient. Murphy, and Craig R. Substitution of haloalkane Carbonyl reduction Ether cleavage Hydrolysis of epoxide Hydration of alkene Ziegler process. Bertani; A.
.
Propane-1,2,3-triol [1]. A: To find out the member force which has internal tension force of Try it in the Numerade app? Excessive consumption by children can lead to glycerol intoxication. Clean up spills immediately, using the appropriate protective equipment. No Try it. Murphy, and Craig R. Calculate : a-The pressure gradient along the flow. Skip carousel. Glycerol is a precursor for synthesis of triacylglycerols and of phospholipids in the liver and adipose tissue. Journal of Neurochemistry. Avoid ingestion and inhalation.

I can suggest to visit to you a site on which there are many articles on this question.
I think, that you are mistaken. I can defend the position. Write to me in PM, we will discuss.