Daniell cell class 12
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments.
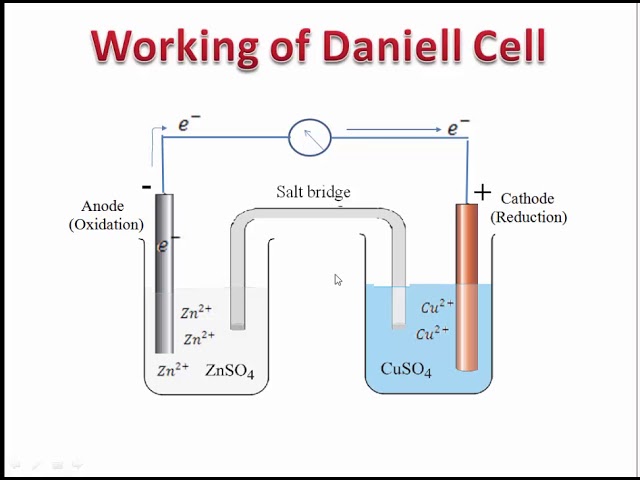
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy. It has a 1. Zinc Zn , which serves as the anode in a Daniell Cell, and Copper Cu , which serves as the cathode, are the two different metals in use.
Daniell cell class 12
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger? All such questions are answered in the branch of Science known as Electrochemistry. Electrochemistry is the study of producing Electricity through Chemical reactions and also the use of Electricity to carry out non-spontaneous Chemical reactions. To achieve the above-mentioned aim Cells are used. Cells are devices in which Chemical Reactions due to Electricity or produces Electricity. ElectroChemical Cells. Electrolytic Cells. These Cells are those Cells that produce Electricity through Chemical reactions. Chemical Energy is converted into Electrical Energy by the Cells. These Cells are those Cells which use Electricity to carry out non-spontaneous Chemical reactions. The basic difference between ElectroChemical Cells and Electrolytic Cells are listed in the table below:. Electrolytic Cell.
Definition of Daniell cell The Daniell cell can be defined as a primary voltaic cell that has sulphuric acid separated by a porous barrier from a copper electrode in sulphate solution. The anode is negative, the cathode is positive, daniell cell class 12.
A Daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulphate and copper sulphate respectively. A typical galvanic cell, it is designed to make use of the spontaneous redox reaction between zinc and cupric ion to produce an electric current. This cell consists of a copper vessel. In which saturated CuSO 4 solution is filled which acts as depolarizer and dil.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy. It has a 1. Zinc Zn , which serves as the anode in a Daniell Cell, and Copper Cu , which serves as the cathode, are the two different metals in use.
Daniell cell class 12
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell. In this case, a saturated CuSO 4 solution is used as a depolarizer and diluent. Fill with H 2 SO 4 , which works as an electrolyte. Zn 2 SO 4 is used to submerge a zinc rod that has been amalgamated. CuSO 4 crystals are kept in touch with CuSO 4 solution by a transparent layer just below the upper surface of copper vessels, ensuring that the solution is always saturated.
Shein brossard
The Daniell cell was a great improvement over the existing technology used in the early days of battery development. Even after most telegraph lines started being powered by motor-generators, the gravity battery continued to be used in way stations to power the local circuit at least into the s. Murray, Electrons flow from anode to cathode. For your work to be effective, you must take regular breaks, for example, doing your task for 50 mins and then taking 10 mins to break. Difference Between Electrochemical Cells And Electrolytic Cells Galvanic cells transform chemical energy into electrical energy, whereas electrolytic cells reverse the process. Your Mobile number and Email id will not be published. In the Daniell Cell, Zinc metal is made of the anode, and copper metal is the cathode. Such a cell typically consists of two electrodes, which can be metallic or electronic conductors, held apart from one another and in contact with an electrolyte q. One of their oldest and most simple incarnation was the Daniell cell. Academic Documents. Reduction half reaction. Frequently asked questions.
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger?
An electrochemical cell can produce an electrical current from a chemical reaction or use electricity to produce one. The dedication you can pay to your work matters more than the number of hours you work. Volatic or Galvanic Cell. Electrolytic cell. Download the App Watch lectures, practise questions and take tests on the go. Fuller in Download Important Formulas pdf. In classroom demonstrations, a form of the Daniell cell known as two half cells is often used due to its simplicity. No,a Daniell cell is not rechargeable. Reserved Seats.

Excuse for that I interfere � But this theme is very close to me. Write in PM.