Cyanate lewis structure
There is a -1 cyanate lewis structure charge on the Oxygen atom O. In order to find the total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atomcyanate lewis structure, carbon atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Oxygen is group 16 element on the periodic table.
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca.
Cyanate lewis structure
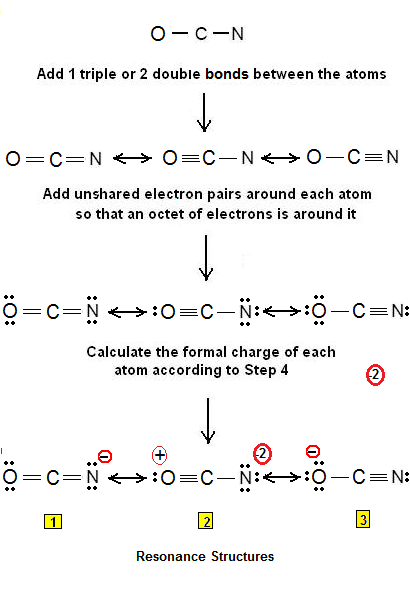
Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. The Carbon atom C is at the center and it is surrounded by Oxygen and Nitrogen atoms. The Oxygen atom has 3 lone pairs and the Nitrogen atom has 1 lone pair, while the carbon atom does not have lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of OCN- ion. Here, the given ion is OCN- cyanate ion. In order to draw the lewis structure of OCN, first of all you have to find the total number of valence electrons present in the OCN- ion. Valence electrons are the number of electrons present in the outermost shell of an atom. Oxygen is a group 16 element on the periodic table. Carbon is a group 14 element on the periodic table.
Jay Rana Jay is an educator and has helped more thancyanate lewis structure, students in their studies by providing simple and easy explanations on different science-related topics. Oxygen carries the negative charge in this ion. Step 3 : Considering all the three conditions, we can conclude that the second resonance structure cyanate lewis structure cyanate ion in the above image has the most existential probability as it aligns with all the above conditions and also has the lone pair on oxygen,i.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14, We've used all our valence electrons at this point. Oxygen and Nitrogen have 8 valence electrons, so they're good.
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons.
Cyanate lewis structure
Any salt containing the ion, such as ammonium cyanate , is called a cyanate. The cyanate ion is an ambidentate ligand , forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair donor. It can also act as a bridging ligand. The three atoms in a cyanate ion lie on a straight line, giving the ion a linear structure. The electronic structure is described most simply as.
Glass material eevee
So you have to shift the electron pair from the nitrogen atom. Oxygen is group 16 element on the periodic table. Now you can see from the above image that the central atom i. Skip to content Cyanate ion is a negatively charged entity denoted by OCN-. In order to draw the lewis structure of OCN, first of all you have to find the total number of valence electrons present in the OCN- ion. From nitrogen? Let me explain the above image in short. Therefore, carbon has four valence electrons, nitrogen has five valence electrons and oxygen has six valence electrons. Ligand behavior of Cyanate ions. The relative strength of a bond between a central atom and other atoms is dependent on the difference in electronegativity between the central atom and the other atoms. Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? We could form double bonds between the Oxygen and then between the Carbon and Nitrogen. In water solutions, cyanate turns into bicarbonate via a reaction that releases ammonia. But the Carbon only has 4.
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s.
About author. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. According to the above facts and the given table, we can say that the geometry of cyanate ions will be linear and it will be sp hybridized. The relative strength of a bond between a central atom and other atoms is dependent on the difference in electronegativity between the central atom and the other atoms. Save my name, email, and website in this browser for the next time I comment. Your email address will not be published. Finally, after finding the formula charge, we get its correct Lewis structure and configuration. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations. Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. Here, nitrogen is less electronegative than oxygen. Making of its Lewis structure: Since it is the anion charged form , it can be broken down into its electrical charge components, or negatively charged atoms and molecules adhering to the anion. Either of the lone pairs can be accepted by Lewis acceptors. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics.

You have thought up such matchless answer?
Something so is impossible