Cuno3 naoh
Direct link to this balanced equation:.
Post by » Tue Oct 05, am. Laurence Lavelle Skip to content. Quick links. Email Link. My question is how to get the net ionic equation for the reaction question a. Not exactly sure how to balance this equation without the Na part. If someone would clarify this question, that would be awesome :.
Cuno3 naoh
I keep rereading what's in my textbook over and over again, but I'm just lost! Could someone please help and explain! Many thanks! You're dealing with a double replacement reaction in which two soluble ionic compounds react to form an insoluble solid that precipitates out of the aqueous solution. Now, notice that you need 2 moles of sodium hydroxide for every 1 mole of copper II nitrate that takes part in the reaction. To get the complete ionic equation , rewrite the soluble ionic compounds as cations and anions. Now, in order to get the net ionic equation , you must eliminate spectator ions , i. Stefan V. Mar 22, Explanation: You're dealing with a double replacement reaction in which two soluble ionic compounds react to form an insoluble solid that precipitates out of the aqueous solution.
Not exactly sure how to balance this equation without the Na part.
.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Cuno3 naoh
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients.
Embroidered flower appliques
Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Question bc Get rid of spectator ions, these are ions that appear the same including same stoichiometric coefficient on both sides of the equation. Reaction stoichiometry could be computed for a balanced equation. I keep rereading what's in my textbook over and over again, but I'm just lost! Chemical forum. Quick links. You can reuse this answer Creative Commons License. Stefan V. Previous Next: balancing chemical equations. Let's balance this equation using the algebraic method. To get the complete ionic equation , rewrite the soluble ionic compounds as cations and anions. Unit converters. If the compound is insoluble, it cannot dissolve in water and will NOT dissociate into its ions.
.
Stefan V. Best For: Redox reactions where electron transfer occurs. My question is how to get the net ionic equation for the reaction question a. Now, both sides have 4 H atoms and 2 O atoms. Not exactly sure how to balance this equation without the Na part. Now, in order to get the net ionic equation , you must eliminate spectator ions , i. If the compound is soluble, it can dissolve in water and thus will dissociate into its ions. I keep rereading what's in my textbook over and over again, but I'm just lost! Balance the changes using electrons: Multiply the number of calcium atoms by 3 and the number of phosphorus atoms by 2. See all questions in Chemical Reactions and Equations. Chemical forum. The limiting reagent row will be highlighted in pink. Related questions Question fee

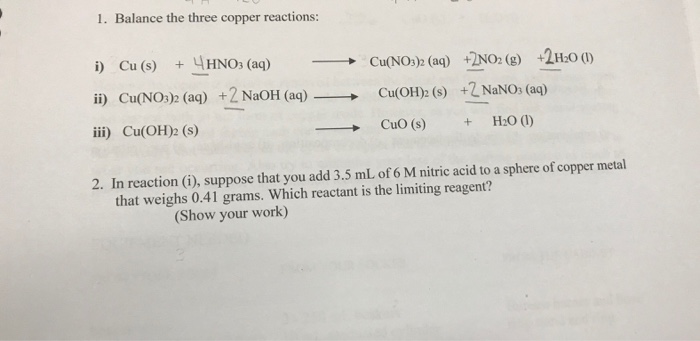
You commit an error. Let's discuss it.
What useful question