Crs cytokine
Federal government websites often end in. The site is secure. Chimeric antigen receptor T cell CART crs cytokine represents a novel and a paradigm-shifting approach to treating cancer.
Together is a new resource for anyone affected by pediatric cancer - patients and their parents, family members, and friends. Cytokine release syndrome CRS is a collection of symptoms that can develop as a side effect of certain types of immunotherapy , especially those which involve T-cells. The syndrome occurs when immune cells are activated and release large amounts of cytokines into the body. However, high levels of cytokines may cause increased inflammation throughout the body. This can be harmful and interfere with a number of body functions. In severe cases, CRS can cause organ failure and even death.
Crs cytokine
Federal government websites often end in. The site is secure. During the last decade the field of cancer immunotherapy has witnessed impressive progress. Highly effective immunotherapies such as immune checkpoint inhibition, and T-cell engaging therapies like bispecific T-cell engaging BiTE single-chain antibody constructs and chimeric antigen receptor CAR T cells have shown remarkable efficacy in clinical trials and some of these agents have already received regulatory approval. However, along with growing experience in the clinical application of these potent immunotherapeutic agents comes the increasing awareness of their inherent and potentially fatal adverse effects, most notably the cytokine release syndrome CRS. This review provides a comprehensive overview of the mechanisms underlying CRS pathophysiology, risk factors, clinical presentation, differential diagnoses, and prognostic factors. In addition, based on the current evidence we give practical guidance to the management of the cytokine release syndrome. Cytokine release syndrome CRS is a systemic inflammatory response that can be triggered by a variety of factors such as infections and certain drugs. Subsequently, CRS has been described after infusion of several antibody-based therapies such as anti-thymocyte globulin ATG [ 3 ], the CD28 superagonist TGN [ 4 ], rituximab [ 5 ], obinutuzumab [ 6 ], alemtuzumab [ 7 ], brentuximab [ 8 ], dacetuzumab [ 9 ], and nivolumab [ 10 ]. CRS has also been observed following administration of non-protein-based cancer drugs such as oxaliplatin [ 11 ] and lenalidomide [ 12 ]. Furthermore, CRS was reported in the setting of haploidentical donor stem cell transplantation, and graft-versus-host disease [ 13 , 14 ]. Cytokine storm due to massive T cell stimulation is also a proposed pathomechanism of severe viral infections such as influenza [ 15 , 16 ]. Lately, with the success of the newer T cell-engaging immunotherapeutic agents there has been a growing interest in CRS since it represents one of the most frequent serious adverse effects of these therapies. T cell-engaging immunotherapies include bispecific antibody constructs and chimeric antigen receptor CAR T cell therapies. Both these immunotherapeutic strategies have recently been carried forward into clinical application and have shown impressive therapeutic activity in several hematologic malignancies, such as acute lymphoblastic B cell leukemia B-ALL , chronic lymphocytic leukemia CLL , and diffuse large B cell lymphoma DLBCL.
Current concepts in the diagnosis and management of cytokine release syndrome. Gauthier J, Turtle CJ.
Daniel W. Lee , Rebecca Gardner , David L. Porter , Chrystal U. Grupp , Crystal L. Mackall; Current concepts in the diagnosis and management of cytokine release syndrome.
Treatment with CAR-T cell therapy and bispecific antibodies eg, blinatumomab for refractory lymphoid malignancies are described separately:. Why UpToDate? Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large.
Crs cytokine
Federal government websites often end in. The site is secure. Chimeric antigen receptor T cell CART therapy represents a novel and a paradigm-shifting approach to treating cancer. CART therapy is associated with unique and potentially life-threatening toxicities, notably cytokine release syndrome CRS. A better understanding of the pathogenesis of CRS is crucial to ensure proper management. In this review, CRS definitions, profiles, risk factors and grading systems are discussed.
Ark dlc
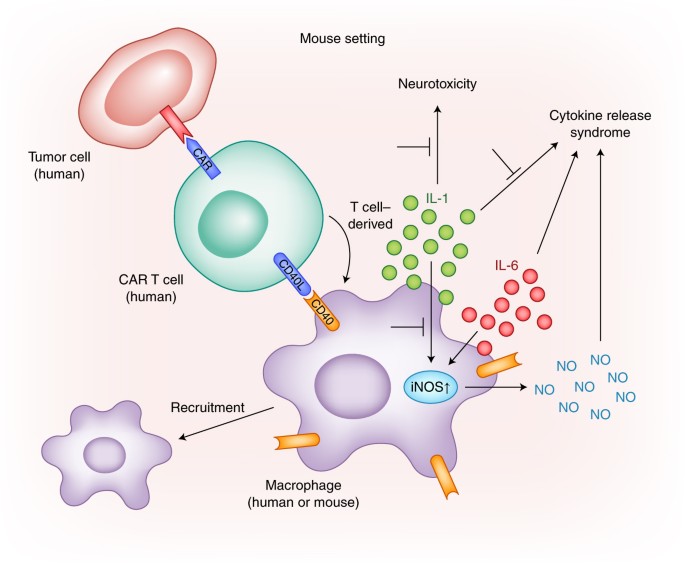
Cancer Discov. Conclusion In the wake of the remarkable success of recently developed immunotherapies the field of immuno-oncology is poised to continue its rapid growth. Lately, with the success of the newer T cell-engaging immunotherapeutic agents there has been a growing interest in CRS since it represents one of the most frequent serious adverse effects of these therapies. These cytokines trigger a chain reaction due to the activation of innate immune cells like macrophages and endothelial cells with further cytokine release. Together with Brentjens et al. Retrieved October 16, Genetically modified T cells in cancer therapy: opportunities and challenges. Monocyte ablation had a negative effect on CAR T cell proliferation and population expansion. ARDS may sometimes require mechanical ventilation. Formulary drug information for this topic. A better understanding of the pathogenesis of CRS is crucial to ensure proper management. The overarching goal of CRS management in patients treated with immunotherapy for cancer is to prevent life-threatening CRS while maintaining the greatest chance for a beneficial antitumor effect.
Thank you for visiting nature.
Clinical studies identified a number of predictors of CRS severity. Box 1 Similarities between cytokine release syndrome, haemophagocytic lymphohistiocytosis and macrophage activation syndrome Haemophagocytic lymphohistiocytosis HLH and macrophage activation syndrome MAS are clinical syndromes of pathological hyperinflammation and uncontrolled macrophage activation that are usually associated with triggers such as viral infections, rheumatological diseases or inherited defects in T cell and natural killer cell functions , , At-risk patients will be monitored for about a month after an immunotherapy infusion. Salama, C. She developed fever on day 1 that peaked at Comment This field is required. Crystal L. Efficacy and toxicity management of 19—28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Rose-John S. References 1. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Philadelphia: W.

Try to look for the answer to your question in google.com
Hardly I can believe that.