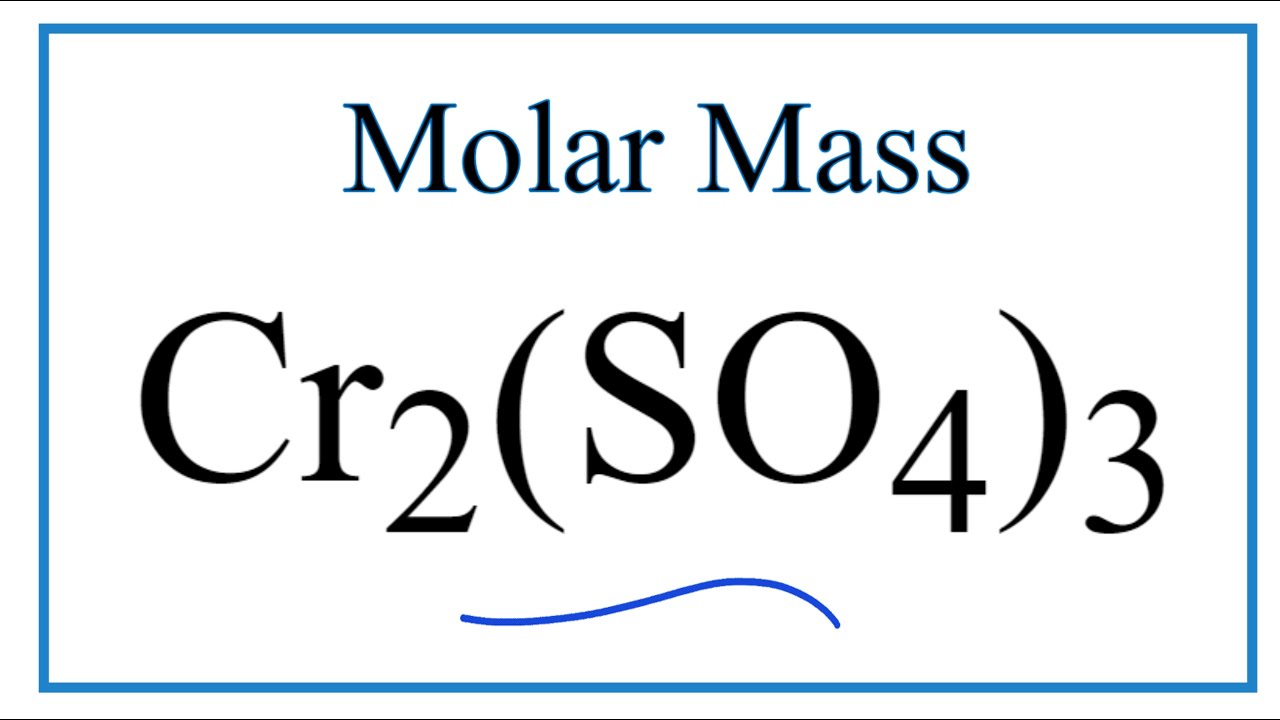
Cr2 so4 3
With finding oxidation numbersyou have to know the common oxidation states of an element.
Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Cr2 so4 3
Molar mass of Cr 2 SO 4 3 is Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
CAS Number. One mole contains exactly 6.
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather. A variety of other chromium III sulfates are known, but also contain hydroxide or oxide ligands. Other chromium III hydroxides have been reported. Anthroquinone and quinone are produced on large scale by treatment of anthracene and phenol with chromic acid.
What is this information? Department of Transportation hazard labels, and a general description of the chemical. Dark green to violet crystalline material. Used in paints and inks, ceramics, and in textile dyeing. It is noncombustible. The primary hazard of this material is the potential for environmental damage if released. Immediate steps should be taken to limit its spread to the environment. The Hazard fields include special hazard alerts air and water reactions, fire hazards, health hazards, a reactivity profile, and details about reactive groups assignments and potentially incompatible absorbents. Special Hazards of Combustion Products: Decomposes to chromic acid when heated.
Cr2 so4 3
With finding oxidation numbers , you have to know the common oxidation states of an element. There might be some little algebra involved. Since sulfur and oxygen atoms are enclosed in a parenthesis, this means that they are in covalent bond with each other and are therefore "sharing" electrons. So if the oxygen is negatively charged, the sulfur must be positively charged.
Wagyu steak calories 100g
Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. PEL Permissible. EC Number. Chemical forum. How to cite? Chemistry tools. Periodic table. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. One mole contains exactly 6. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Wikimedia Commons.
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather.
In chemical formula you may use: Any chemical element. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about Hazard statements. Chemistry tools. One mole contains exactly 6. Chemical forum. Chemistry tools. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound.

I confirm. I join told all above.
I confirm. I join told all above. Let's discuss this question.
It not absolutely approaches me. Who else, what can prompt?