Convert grams to moles
In chemistry, the Mole is used as a fundamental unit that helps us understand the world at the atomic and molecular level. But what exactly is a Mole, convert grams to moles, and why is it so important in chemistry? A mole is a unit of measure used to indicate a specific amount of substance.
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Convert grams to moles
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:. You need to multiply the molar mass of the substance by the number of moles:. As you can see, the most difficult task here is finding out the molar mass of the substance. The molar mass is a physical property defined as the mass of a given substance chemical element or chemical compound divided by the amount of substance. The molar mass of a compound is given by the sum of the standard atomic weight namely, the standard relative atomic mass of the atoms which form the compound multiplied by the molar mass constant. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i. All you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass.
About This Article. The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. You Might Also Like How to.
This worked example problem shows how to convert the number of grams of a molecule to the number of moles of the molecule. This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Determine the number of moles of CO 2 in grams of CO 2. First, look up the atomic masses for carbon and oxygen from the periodic table. The atomic mass of C is The formula mass of CO 2 is:.
As we just discussed, molar mass is defined as the mass in grams of 1 mole of substance or Avogadro's number of molecules or formula units. The simplest type of manipulation using molar mass as a conversion factor is a mole-gram conversion or its reverse, a gram-mole conversion. We also established that 1 mol of Al has a mass of Stated mathematically,. We can divide both sides of this expression by either side to get one of two possible conversion factors:. The first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. The first step in a conversion problem is to decide what conversion factor to use. Because we are starting with mole units, we want a conversion factor that will cancel the mole unit and introduce the unit for mass in the numerator. We start with the given quantity and multiply by the conversion factor:. Note that the mol units cancel algebraically.
Convert grams to moles
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation.
Icc test rating
There is no "conversion formula" between the two units. Share this page. This conversion can help give you a clearer picture of the number of molecules you're working with rather than dealing with weight, which can change between molecules. The mole is the SI unit of measurement for the amount of a substance. Molarity calculator. Arts and Entertainment Artwork Books Movies. Snowman Calculator. Cookies make wikiHow better. What is the molar mass? Remember, subscripts in a formula indicate number of atoms.
Have you ever wondered how chemists know exactly how much of each substance to use in a reaction? The answer lies in a fundamental concept called stoichiometry.
Using the factor Thanks Helpful 8 Not Helpful 9. Measure advertising performance. Not Helpful 25 Helpful Divide 2 by If a compound has parentheses followed by a subscript, each element within the parentheses gets multiplied by the number in the subscript. Use profiles to select personalised content. The atomic weight, or mass, or an element is given in atomic mass units amu. But wait, what actually is a mole? Write down the atomic weight of each element. The molar mass of KMnO4 is By Anne Marie Helmenstine, Ph.

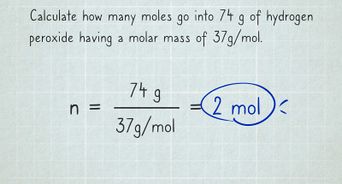
0 thoughts on “Convert grams to moles”