Compound containing both ionic and covalent bonds
Last updated on Jan 7, Get Started.
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom.
Compound containing both ionic and covalent bonds
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bonds , on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions. Many of these compounds contain a metal, a nonmetal, and also hydrogen. However, other examples contain a metal joined via an ionic bond to covalently bonded nonmetals. Here are examples of compounds that exhibit both types of chemical bonding:. In ammonium sulfide, the ammonium cation and the sulfide anion are ionically bonded together, even though all of the atoms are nonmetals. The electronegativity difference between ammonium and the sulfur ion allows for an ionic bond. At the same time, the hydrogen atoms are covalently bonded to the nitrogen atom. Calcium carbonate is another example of a compound with both ionic and covalent bonds. Here calcium acts as the cation, with the carbonate species as the anion.
Rajasthan Gram Vikas Adhikari. Rajasthan Pre D.
.
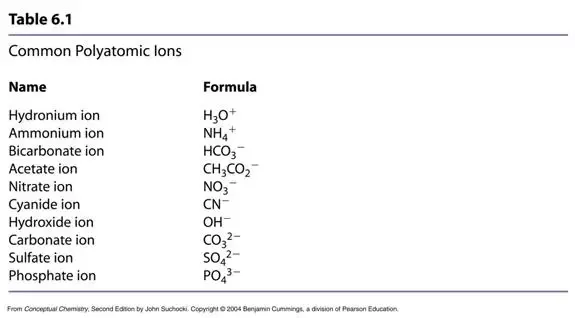
What elements make covalent bonds? Covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Thus, the compound formed from sodium and chlorine will be ionic a metal and a non-metal. Nitrogen monoxide NO will be a covalently bound molecule two non-metals , silicon dioxide SiO 2 will be a covalently bound molecule a semi-metal and a non-metal and MgCl 2 will be ionic a metal and a non-metal. The ammonium ion see figure below consists of one nitrogen atom and four hydrogen atoms.
Compound containing both ionic and covalent bonds
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions Figure 2. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table.
Office depot ermita
MP Cooperative Bank Clerk. Here are examples of compounds with both ionic and covalent bonds. HP TET. Telangana High Court Copyist. MP Police SI. West Bengal Sub Assistant Engineer. NDA Group C. NVS Group C. Telangana High Court Examiner. Telangana High Court Record Assistant.
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom.
NVS Group C. Haryana Police. Maharashtra Forest Guard. Odisha Junior Teacher. UP Police Head Operator. RPF Constable. Hence the correct answer to the question is NH 4 Cl containing both ionic and covalent bonds. EMRS Accountant. RPSC Programmer. Testbook Edu Solutions Pvt. Usually, all you have to do to predict the type of chemical bond between two atoms is compare their electronegativity values. RCFL Technician.

Precisely, you are right
You commit an error. Write to me in PM, we will discuss.
You are similar to the expert)))