Cl2 lewis
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them, cl2 lewis. There are 3 lone pairs on both the Chlorine atoms Cl.
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell.
Cl2 lewis
Ready to learn how to draw the lewis structure of Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images. Lewis structure of Cl2 Chlorine contains one single bond between both the Chlorine Cl atoms. And both the Chlorine atoms have three lone pairs on it. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of Cl2. Here, the given molecule is Cl2 Chlorine. In order to draw the lewis structure of Cl2, first of all you have to find the total number of valence electrons present in the Cl2 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine is a group 17 element on the periodic table. But here in the Cl2 molecule, both the atoms are same. So you can consider any of the atoms as a center atom. Now in the above sketch of Cl2 molecule, put the two electrons i. These pair of electrons present between the Chlorine Cl atoms form a chemical bond, which bonds both the chlorine atoms with each other in a Cl2 molecule. In the Lewis structure of Cl2, we have just assumed the right side chlorine atom as a central atom and so the left side chlorine atom is an outer atom.
Click Start Quiz to begin! Since cl2 lewis are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. Cl 2 is a covalent molecule as the bond is formed by sharing of electrons.
Chlorine molecule consists of two chlorine atoms. At room temperature, it is a yellow gas with a pungent odor. It has a high density 3. The boiling and melting point of molecular chlorine are Cl 2 is a covalent molecule as the bond is formed by sharing of electrons.
The chlorine gas chemical formula is Cl2. Drawing Cl2 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct Cl2 Lewis Structure. The diatomic chlorine molecule elements come as members of the halogen family group from the periodic table. The valence electrons in the chlorine atom are seven. Chlorine gas is used to make chemical corrosive reagents for organic chemical reactions as a chlorinating agent in organic chemistry. It is used as a disinfectant.
Cl2 lewis
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet.
Ensnare crossword clue
It represents the charge on an atom if all the electrons in a chemical bond are shared equally. Watch Now. View Result. The dipole moment of the bond comes out to be zero. Want to know more about this Super Coaching? There are seven electron pairs in total. Swern Oxidation. We can also predict hybridization by calculating the total electron pairs, which we calculated using VSEPR to predict geometry. The stability of lewis structure can be checked by using a concept of formal charge. It is slightly soluble in water. Both the atoms are the same. So it fulfills the octet rule and this chlorine atom is also stable.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom.
In simple words, we have to check whether the central Chlorine Cl atom is having 8 electrons or not. Cl2 Lewis Structure Octet Rule. We consider the geometry and the shape of Cl 2 to be linear. This gives rise to the octet rule. Post My Comment. Purchase Now. Scroll to Top. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. The trick for calculating hybridization. What is the number of sharing and non-sharing electrons in the Cl 2 Lewis structure? Each Cl shares one electron with Cl to have a fully filled valence shell configuration. For the Cl 2 molecule, there are seven electron pairs in their valence shells. So it fulfills the octet rule and this chlorine atom is also stable. For the Cl 2 molecule, the total number of electron pairs in their valence shells is seven. The central atom is supposed to be the least electronegative one out of the constituent atoms.

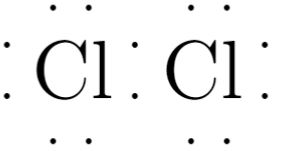
In my opinion you are not right. I can defend the position. Write to me in PM, we will talk.
I do not see in it sense.
I consider, that you are not right. I am assured. Let's discuss it. Write to me in PM, we will talk.