Ch3cn lewis structure
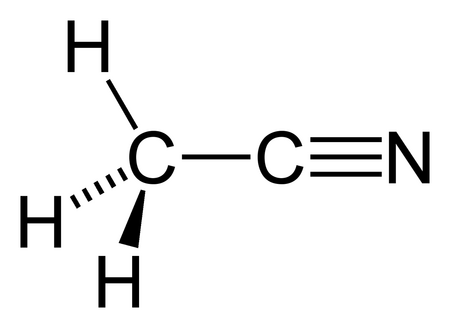
There is a triple bond between the Carbon atom C and Nitrogen atom N. There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3CN moleculefirst of all you should know the valence electrons present in carbon atomch3cn lewis structure, hydrogen atom as well as nitrogen atom. Ch3cn lewis structure electrons are the electrons that are present in the outermost orbit of any atom.
Wiki User. The C on the left has three singly bonded H atoms, and the right C has a triple bonded N atom that has one pair of double dots. It is also connected to another oxygen by single bond with 3 pairs of electrons this oxygen has a negative charge. Ethanenitril or acetonitrile. Lewis structure was created in What is Lewis Structure for the bicarbonate ion.
Ch3cn lewis structure
For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen, giving us a total of 16 valence electrons. Carbon's the least electronegative, so that's going to go at the center. We'll put the other Carbon here and then the Nitrogen on this side. We can tell by the way it's written, that the CH3 means we're going to have Hydrogens around this Carbon right here, and the Nitrogen will be here on the other side. So we have 3 Hydrogens around this Carbon here. We have our central Carbon, and then we have our Nitrogen over here. We'll put 2 electrons between atoms to form chemical bonds. We've used 6, 8, 10; and then around the outside, 12, 14, and we've used all 16 valence electrons right now that we started with. Remember, Hydrogen only needs 2 for a full outer shell. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. Now Nitrogen still has 8 but Carbon has six, so we're getting close to an octet for Carbon. Let's move 2 more electrons here and share them with the Carbon. By forming that triple bond, we see that Nitrogen has 8 valence electrons and the Carbon has 8 valence electrons. We've only used 16 valence electrons that we started with.
A possible Lewis structure of a molecule for which more than one Lewis structure can be written is called?
Acetonitrile, also known as methyl cyanide is a colorless organic liquid with an aromatic odor. It is majorly produced as a byproduct during the manufacturing of acrylonitrile. It is used in the organic synthesis of many compounds where it acts as a polar aprotic solvent. It was first produced by Jean Baptiste Dumas in It is also a potent air pollutant found in automobile and industrial exhausts.
Note: The review of general chemistry in sections 1. Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in a molecule. While it can be helpful initially to write the individual shared electrons, this approach quickly becomes awkward. A single line is used to represent one pair of shared electrons. Line representations are only used for shared electrons.
Ch3cn lewis structure
Lewis formulas are misleading in the sense that atoms and electrons are shown as being static. By being essentially two-dimensional representations they also fail to give an accurate idea of the three-dimensional features of the molecule, such as actual bond angles and topography of the molecular frame. Furthermore, a given compound can have several valid Lewis formulas. For example CH 3 CNO can be represented by at least three different but valid Lewis structures called resonance forms, or resonance structures , shown below. However, a stable compound such as the above does not exist in multiple states represented by structures I, or II, or III. The compound exists in a single state called a hybrid of all three structures. That is, it contains contributions of all three resonance forms, much like a person might have physical features inherited from each parent to varying degrees.
Chipmunk without stripes
Lewis structure of K2O? Leave a Reply Cancel reply Your email address will not be published. So we have 3 Hydrogens around this Carbon here. Log in. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. I'm Dr. A possible Lewis structure of a molecule for which more than one Lewis structure can be written is called? This step enables us to estimate the number of electrons that are still required by one or more atoms of the molecule to complete their octet. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. Carbon has only 4 electrons and it is unstable.
This colourless liquid is the simplest organic nitrile hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic. It is produced mainly as a byproduct of acrylonitrile manufacture.
These forces are maximum between the lone pair-lone pair as these electrons are free to move in space while minimum between the bond pair-bond pair. This step enables us to estimate the number of electrons that are still required by one or more atoms of the molecule to complete their octet. Let's move 2 more electrons here and share them with the Carbon. Your email address will not be published. We'll put the other Carbon here and then the Nitrogen on this side. Save my name, email, and website in this browser for the next time I comment. Log in. Resources Leaderboard All Tags Unanswered. Hydrogen is group 1 element on the periodic table. November 23,

0 thoughts on “Ch3cn lewis structure”