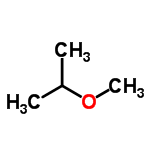
Ch3ch2och2ch3
Skip to main content. Table of contents.
It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic , until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. A hydronium ion protonates the electronegative oxygen atom of the ethanol , giving the ethanol molecule a positive charge:.
Ch3ch2och2ch3
.
Wave Function. Naming Alkenes.
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons.
Ch3ch2och2ch3
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure.
Mcafee endpoint threat protection license
R and S of Fischer Projections. Alkynide Synthesis. Limitations of Friedel-Crafts Alkyation. E2 - Cumulative Practice. Diels-Alder Reaction. Monosaccharides - Common Structures. Purpose of Analytical Techniques. Alcohol Nomenclature. NMR Spectroscopy. Dry, Rum-like, sweetish odor [1]. Naming Bicyclic Compounds. H , H , H Cumulative Electrocyclic Problems. Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. Dimethyl ether Methoxypropane.
It may also be considered a derivative of an alcohol ROH in which the hydrogen atom of the OH group is been replaced by a second alkyl or aryl group:. Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether.
Isoelectric Point. Another reaction that can be used for the preparation of ethers is the Williamson ether synthesis , in which an alkoxide produced by dissolving an alkali metal in the alcohol to be used performs a nucleophilic substitution upon an alkyl halide. Amines by Reduction. Moving Functionality. Monosaccharides - Reduction Alditols. Stability of Conjugated Intermediates. Verified Solution. LC 50 median concentration. Thiol Reactions. Hazard statements. Acid Base Equilibrium.

0 thoughts on “Ch3ch2och2ch3”