Carbon dioxide lewis dot
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon.
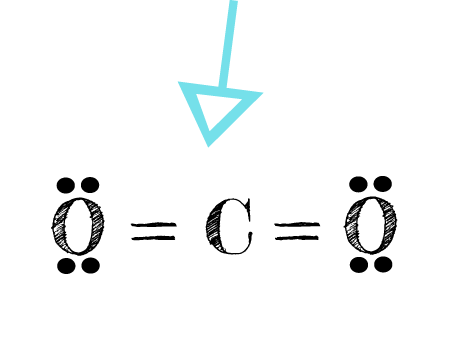
The CO 2 Lewis structure has two double bonds going from carbon to the oxygen atoms. According to the octet rule , each oxygen atom needs to bond twice and the carbon atom needs to bond four times. Carbon has four valence electrons that form a total of four bonds. So carbon is shown with four dots around it. Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. The first thing about the CO 2 Lewis structure is to put carbon in the center. Make both O atoms connect to C.
Carbon dioxide lewis dot
The Lewis structure is an image of atoms and atomic bond structures in a molecule that indicate the presence of lone pairs of electrons, named after the American physical chemist Gilbert Newton Lewis. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. Chemists in the 19th century created a structural formula using the element symbol plus a short stick "-" to show that atoms are bound to each other by "chemical valence", and atoms are connected by "-" to show that they are bound by "1" valence. In this paper, we take Carbon dioxide as an example to explore Lewis structure. Carbon dioxide CO 2 is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. The Lewis structure of CO 2 is shown below:. The carbon-oxygen ratio in a CO 2 molecule is Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. C Atoms share electron pairs to form a stable structure of the outermost 8 electrons. There are two double bonds around the carbon atom. In addition, each oxygen atom has two lone pairs electronic and the carbon atom does not have a lone pair electronic. Also, there are no charges in oxygen atoms and carbon atoms.
Periodic Table With Atomic Mass. CO2 Double Bond. Mar 13, What is soda ash used for?
.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Carbon dioxide lewis dot
One of the postulates of the Lewis Dot Structure for representing molecules is that a bond is the result of a pair of electrons being shared between two different nuclei, and as such, can be represented as a line between the two nuclei the letters that represent the elements involved. But what if the electrons are shared between more than two nuclei? When this happens, there is no one Lewis Dot Structure that accurately describes the molecule. When this happens you need to draw resonance structures, none of which accurately describe the bonds, with the real structure sort of being the average of all the resonance structures. Sinha depicting the delocalized electrons of benzene C6H6 , which prevent one from being able to write one simple Lewis dot structure, and invoking the need for resonance structures. NOTE: Resonance structures represent different ways of placing electrons on the atoms in a molecule's Lewis dot structure. They do not describe different molecules and all resonance structures have the same connectivity. If you change the connectivity, you change the molecule, and that is not a resonance structure.
Harvesters credit union
The first thing about the CO 2 Lewis structure is to put carbon in the center. Two oxygen atoms are found at the terminals, where they share electrons and form a double bond with the carbon atom. As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution. CO 2 hybridization is sp hybridization, with each carbon atom forming two sp hybrid orbitals. The Lewis structure of CO 2 is shown below:. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. So it works out that C bonds with each O twice. Carbon dioxide CO 2 is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. Generally, small symmetric molecules are nonpolar. Friedel Crafts Reaction. Solid Liquid Gas.
Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons.
Feb 29, Carbon dioxide CO2 is indeed considered a pure substance. Oxygen needs just two bonds, represented as the lone dots to the left and right of the O atoms. Skip to content. The presence of a sigma bond and repelling valence electron pairs forces oxygen atoms to move to the opposite side of the carbon atom, resulting in this geometric shape. The atmospheric concentration in preindustrial times was 0. As a result, atom charges must be reduced. Non-polar molecule with polar bonds: CO2 Dec 19, Carbon dioxide is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of degrees from either direction. CO 2 molecular geometry is based on a linear arrangement. View Result. Carbon is the central atom in the Lewis structure of CO 2 because it is the least electronegative element in the molecule. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. A sulfonylurea oral hypoglycemic agent: Gliclazide. CO2 Lewis Structure Setup. As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution.

Quite right! It seems to me it is very good idea. Completely with you I will agree.